ABSTRACT
The present study was designed for a phylogenetic tree analysis of Campylobacter species as molecular method for genetic identification of Campylobacter jejuni from human and birds sources and birds and amplified by polymerase chain reaction assay using specific primers for 16S rRNA gene of Campylobacter jejuni (GenBank: EF136575.1). In this study, the multiple sequence alignment analysis and neighbor joining phylogenetic tree analysis was performed by using Unweight Pair Group method with Arithmetic Mean (UPGMA tree) in MEGA 6.0 version that analyzes 827 bp for ribosomal 16S rRNA gene. C. jejuni was detected in 40% (16/40) of stool samples collected from patients suffering from gastroenteritis, while detection rates of C. jejuni were 15% (3 /20) and 10% (2/20) of fecal samples of domestic chicken and pigeon respectively by PCR assay. The phylogenetic analysis results revealed that all local isolates of Campylobacter spp. were closed related to NCBI-Blast C. jejuni strain No.Y19244.1, whereas other NCBI-Blast Campylobacter spp. were out of tree and more different to ten Campylobacter spp. Iraq isolates and also found the relationships between the local isolates of Campylobacter spp. (Human, Domestic chicken, and Pigeon). This study represents the first report on the use of molecular phylogeny to Campylobacter spp. obtained in Iraq and confirmed the zoonotic potential of C. jejuni.
Key words: Phylogenetic tree, Campylobacter species, 16S ribosomal gene, human, birds, Iraq.
Campylobacteriosis is a common zoonotic disease that affect human and cause gastrointestinal disturbances (Barakat et al., 2013). Campylobacter jejuni is responsible for 90% of Campylobacter species human infections and they occur in sporadic way (Schielke et al., 2014). Campylobacter is one of the most frequently occurring bacterial agents of gastroenteritis in human (WHO, 2012). Most bird flocks are colonized within several days and still so until slaughter. The handling and ingesting of contaminated meat with Campylobacter, especially poultry meat is considered an important source of food-borne gastroenteritis in human (Hermans et al., 2011).
Today, attention has turned to nucleic acid technology; the polymerase chain reaction (PCR) and related techniques are rapid, specific and sensitive as compared to other tests used in detection of Campylobacter spp. (Englen and Kelley, 2000). C. jejuni is the Campylobacter spp. predominantly found in infected humans and colonized broilers. Sequence analysis of the 16S rRNA gene is very useful for identification of bacteria to genus and species level (Hansson et al., 2008). The potential application of the 16S rRNA gene for determining phylogenetic relationships among all living organisms had attracted much interest and would play a major role in extensive rearrangement of Campylobacter taxonomy (Woese, 1987).
Phylogenetic analysis may be used as a molecular tool in future studies in the surveillance of Campylobacter-like organisms (Nayak et al., 2014). C. jejuni is isolated from stool samples of diarrheic children and confirmed phenotypically on the basis of biochemical tests in many provinces in Iraq (Saliih and Al-Saad, 1994; Mohammad et al., 2004; Al-Ani et al., 2008). C. jejuni is identified by conventional PCR assay in human and domestic chicken in Al-Qadissiya province, Iraq (Al-Hisnaway, 2008). Abd (2014) proved that the detection rate of C. jejuni in human was 55.2% by Real-Time PCR Assay in Al-Muthanaa province, Iraq. The present study aimed at examining and analyzing the partial 16S ribosomal RNA gene sequence for construction of phylogenetic trees analysis of Campylobacter spp. Iraq isolates from infected humans, domestic chicken and pigeons in comparison to those of other NCBI-Blast Campylobacter spp.
Samples collections
Human stool samples
A total of 40 stool samples of patients suffering from enteritis with ages ranging from 1 to 50 years were collected from general hospital in Al-Qadissiya province, Iraq during a period 6 months from October 2014 to March 2015 and after clinician consultation (included diarrhea, symptoms comprising vomiting, abdominal pain, fever) and microscopically examination in the hospitals where many samples contain motile bacteria, pus and few contain mucous and blood.
Bird samples
Fresh fecal samples were collected randomly from 20 flocks of domestic chicken from different farms, as well as 20 fecal samples of pigeon were collected from the same farms in Al-Qadissiya province, Iraq. The samples were collected during a period 6 months from October 2014 to March 2015.All samples were placed in test tube containing 3 ml of peptone water in sterile condition and were immediately transported to the laboratory during 3 to 6 h in a cooler with ice packs. All the samples were frozen at -20°C for DNA extraction.
Genomic DNA extraction
Genomic DNA was extracted from stool samples by using AccuPrep® Stool DNA Extraction Kit, Bioneer, Korea. The extraction was done according to company instructions. After that, the extracted gDNA was checked by Nanodrop spectrophotometer, store in -20°C in refrigerator until perform PCR.
Polymerase chain reaction (PCR)
PCR assay was carried out by using specific primer which was designed in this study from highly conserved regions of 16S ribosomal of C. jejuni (GenBank: EF136575.1). 16SrRNA forward primer (CGCACGGGTGAGTAAGGTAT) and 16SrRNA reverse primer (TAAACACATGCTCCACCGCT) were provided by Bioneer company, Korea and using DNA C. jejuni as positive control and it was provided by Genekam, Germany. PCR master mix was prepared by using AccuPower® PCR PreMix kit Bioneer, Korea. The PCR premix tube contains freeze-dried pellet of Taq DNA polymerase 1U, dNTPs 250 µM, Tris-HCl (pH 9.0) 10 mM, KCl 30 mM, MgCl2 1.5 mM, stabilizer, and tracking dye and the PCR master mix reaction was prepared according to kit instructions in 20 µl total volume by adding 5 µl of purified genomic DNA and 1.5 µl of 10 pmole of forward primer and 1.5 µl of 10 pmole of reverse primer, then complete the PCR premix tube by deionizer PCR water into 20 µl and briefly mixed by Exispin vortex centrifuge (Bioneer. Korea). The reaction was performed in a thermocycler (Mygene Bioneer. Korea) by set up following thermocycler conditions; initial denaturation temperature of 95°C for 5 min; followed by 30 cycles at denaturation 95°C for 30 s, annealing 58°C for 1 min, and extension 72°C for 1 min and then final extension at 72°C for 10 min. The 827 bp PCR products were examined by electrophoresis in a 1% agarose gel, stained with ethidium bromide, and visualized under UV trans-illuminator.
DNA sequencing method
The 827 bp PCR product was purified from agarose gel by using EZ EZ-10 Spin Column DNA Gel Extraction Kit, Biobasic. Canada. The purified 16S rRNA gene PCR product samples were sent to Bioneer Company in Korea to perform the DNA sequencing using 16SrRNA forward primer by AB DNA sequencing system. DNA sequencing method was performed for confirmative Phylogenetic tree relationship analysis of Campylobacter spp. based on 16S ribosomal RNA gene by Phylogenetic tree analysis using Unweight Pair Group method with Arithmetic Mean (UPGMA tree) in MEGA 6.0 version.
Identification of C. jejuni in human and birds by PCR assay
Campylobacter is considered as human pathogen despite of it commensal organisms in domestic poultry and livestock. The present study describes a molecular method for detection C. jejuni from human and bird sources by using specific primer of 16S ribosomal of C. jejuni (Figure 1). Polymerase chain reaction (PCR) analysis using Campylobacter genus-specific partial 16S rRNA primers revealed the presence of Campylobacter spp. DNA in the faces (Turowski et al., 2014), where conventional PCR is rapid as nearly 2 times and sensitive method to determine Campylobacter spp. in comparison with culturing and this enhance its application as timesaving method of Campylobacter spp. by using 16SrRNA gene primer (Stoyanchev, 2004). Zhang et al. (2013) proved that PCR assay was sensitive (100%) in comparison with (49%) sensitivity of direct bacterial culture.
In present study, detection rate of C. jejuni in human was 16 (40%) out of (40) stool samples collected from infected patients which suffered from diarrhea and some of them suffered other symptoms such as fever, colic and vomiting. This result shows that detection rate of C. jejuni in human was relatively low, compared with the results of other studies reported in Iraq describing Campylobacter in human, which reported that the prevalence of campylobacteriosis was 55.2 and 66.7% (Abd, 2014; Al-Amri et al., 2007), while this result was higher than that record by Al-Hisnaway (2008) who found C. jejuni in 33.3% of stool samples of human by PCR assay in Al-Qadissiya province in Iraq. The different detection rate of the present study in comparison with other studies may influence many factors such as age, season, geography and immune state of human.
In this study, 3/20 (15%) fecal samples of domestic chicken were identified as C. jejuni by using specific primers of 16S ribosomal of C. jejuni by PCR assay and this result agree with Al-Hisnaway (2008) who detected C. jejuni with 17.6% from chicken fecal samples by conventional PCR assay, where C. jejuni has been reported to be the most frequent species recovered from poultry and poultry carcasses (Jorgensen et al., 2002).
The occurrence of C. jejuni in pigeon feces has been studied in several countries worldwide. In the present study, the detection rate of C. jejuni in pigeons fecal samples was 10% (2/20) (Table 1), this result was lower than that record by Casanovas et al. (1995) who found Campylobacter spp. in 26.2% of fecal pigeon samples and all of Campylobacter species isolated from pigeon fecal samples was C. jejuni (100%).

Sequencing analysis of 16S rRNA genes of Campylobacter spp. Iraqi isolates
The partial sequences for 16S ribosomal RNA genes of ten Iraq isolates Campylobacter spp. can be found under the accession numbers at NCBI-Gen Bank submission and they are shown in Table 2. Sequence analysis of ten samples positive for Campylobacter spp. was performed to confirm the PCR results in this study, the DNA sequencing analysis of 16S rRNA gene 827 bp PCR product by multiple sequence alignment Unweight Pair Group method with Arithmetic Mean (UPGMA tree) in MEGA 6.0 version showed specific detection of C. jejuni. These studies agreed with Dewhirst et al. (2005) who identified representing either C. jejuni or Campylobacter coli by 16S rRNA sequence analysis.
The nucleotide sequences of the 16S rRNA genes of ten Campylobacter spp. Iraq local isolates of human and birds were determined and compared with 16S rRNA sequences of eight strains of Campylobacter spp. The results showed that the sequence identity was 99% between ten Campylobacter spp. Iraq local isolates and C. Jejuni (Y19244.1), Campylobacter subantarctic (AM. 933373.1), C. subantarctic (AM. 933374.1) and C. coli (AM.042699.1) (Tables 3 and 4). The bacteria with relatively small genomes, such as C. jejuni may undergo genetic variation to increase their potential to adapt to new environments; such genotypic variation could result in phenotypic changes. These variations are probably important in the transmission route from broiler to man, where Campylobacter spp. must survive several hostile environments (Hansson et al., 2008).


Phylogenetic analysis
Phylogenetic tree analysis based on the clone 16S rRNA gene, partial sequence used for confirmative detection of Campylobacter spp. Iraq
isolates that included this study where phylogenetic analysis of 16S rRNA gene sequences has become the primary method for determining prokaryotic phylogeny. Therefore, the validity of 16S rRNA gene based phylogenetic analyses is of fundamental importance for prokaryotic systematics (Dewhirst et al., 2005). However, studies have suggested that multiple strains should be investigated to evaluate the degree of sequence diversity within and between species (Clayton et al., 1995). In the present study, the phylogenetic tree was constructed based on the ten Campylobacter spp. Iraq isolates included {(n=5) human, (n=3) chicken and (n=2) pigeons} and nine strains of NCBI-Blast Campylobacter spp.
The ten Campylobacter spp. Iraq isolates showed close relationship with NCBI-Blast C. jejuni (Y19244.1) compared to other strains of NCBI-Blast Campylobacter spp. (Figure 2). These results agreed with Weis et al. (2014) who used phylogenetic analyses of 16S rRNA sequence data to distinguish C. jejuni from other species and to map strains found in crows with strains previously isolated from humans, livestock, and poultry. Nayak et al. (2014) referred to phylogenetic analysis providing a rapid, accurate and effective method for identification of species within the Campylobacter.


Host relationship analysis of Campylobacter spp. Iraq strains
In present study, we have investigated putative specificity of the host using phylogenetic analysis of genetically closely related Campylobacter spp. from different sources where recent studies have suggested a potential role for birds in zoonotic transmission of Campylobacter spp., the leading cause of gastroenteritis in humans worldwide (Petersen et al., 2001; Broman et al., 2004; Weis et al., 2014). The results showed Campylobacter spp. IQH-2(KR133491.1) and Campylobacter spp.IQH-3(KR133492.1) isolates of human were more close relationship with Campylobacter spp.IQP-1 (KR133488.1) and Campylobacter spp.IQP-2 (KR133489.1) isolates of pigeons, as well as with Campylobacter spp.IQDC-1(KR133485.1) and Campylobacter spp.IQDC -3(KR133487.1) isolates of domestic chickens. Campylobacter spp. IQH-1(KR133490.1), Campylobacter spp.IQH-4 (KR133493.1) and Campylobacter spp.IQH-5 (KR133494.1) of human were close related with Campylobacter spp.IQDC-2 (KR133486.1) of domestic chicken (Figure 3). These results agreed with Schouls et al. (2003) who record about 75% of the human strains were found to be most closely related to the patterns of the other human strains, and the patterns of 20% of the human strains were more similar to the patterns of the strains isolated from poultry.
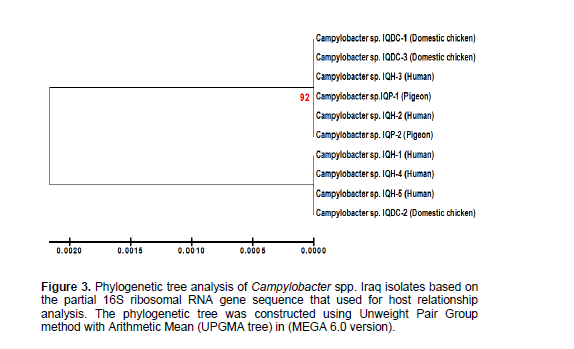
This study suggested that phylogenetic tree analysis is based on 16S ribosomal RNA gene, partial sequence can be used for confirmative detection of Campylobacter spp. isolates and determine the close relationship between Campylobacter spp. isolated from human, domestic chicken and pigeon. These results highlighted the importance of domestic chicken and pigeon as a potential source of human Campylobacter gastroenteritis.
The author has not declared any conflict of interests.
REFERENCES
|
Al-Muthanna Province. Msc. thesis, Al-Qadissiya. University, Iraq.
|
|
|
|
Al-Amri A, Senok A C, Ismaeel A Y, Al-Mahmeed A, Botta GA (2007) . Multiplex PCR for Direct Identification of Campylobacter spp. in Human and Chicken Stools. J. Med. Microbiol. 56:1350-1355.
Crossref
|
|
|
|
Al-Ani MM, Ali FJ, Al-Mawla SO, Ahmed RK (2008). The role of Campylobacter species in diarrhea among children under fve years of age in Ramadi City, west of Iraq. Al-Anb. Med. J. 6(1):76-87.
|
|
|
|
Al-Hisnaway ZF (2008). Detection of Campylobacter spp. in broiler as a Source of children diarrhea using PCR technique in Al-Diwanyiah Governorate. Msc. thesis, Al-Qadissiya. University, Iraq.
|
|
|
|
Barakat AMA, Rabie NS, Zaki MS (2013). Bio-Surveillance of Campylobacteriosis as food borne illness in Egypt by Recent Accurate Diagnostic Methods. Life Sci. J. 10(3):1528-1533.
|
|
|
|
Broman T, Waldenström J, Dahlgren D, Carlsson I, Eliasson I, Olsen B. (2004). Diversities and similarities in PFGE profiles of Campylobacter jejuni isolated from migrating birds and humans. J. Appl. Microbiol. 96:834-843.
Crossref
|
|
|
|
Casanovas LM, De Simón MD, Ferrer JA , Monzón G (1995). Intestinal carriage of Campylobacters, Salmonellas, Yersinias and Listeria in pigeons in the city of Barcelona. J. Appl. Microbiol. 78:11-13.
Crossref
|
|
|
|
Clayton RA, Sutton G, Hinkle PS, Jr Bult C, Fields C (1995). Intraspecific variation in small-subunit rRNA sequences in GenBank: Why single sequences may not adequately represent prokaryotic taxa. J. Appl. Microbiol.45:595-599.
Crossref
|
|
|
|
Dewhirst FE, Shen Z, Scimeca MS, Stokes L (2005). Discordant 16S and 23S rRNA Gene Phylogenies for the Genus Helicobacter: Implications for Phylogenetic Inference and Systematics. J. Bacteriol. 187(17):6106-6118.
Crossref
|
|
|
|
Englen MD, Kelley LC (2000).A rapid DNA isolation procedure for the identification of Campylobacter jejuni by the polymerase chain reaction PCR. Lell. Appl. Microbiol. 31:421-426.
Crossref
|
|
|
|
Hansson I, Persson M, Svensson L, Engvall EO, Johansson K (2008). Identification of nine sequence types of the 16S rRNA genes of Campylobacter jejuni subsp. jejuni isolated from broilers. Acta. Vet. Scand. 50:10.
Crossref
|
|
|
|
Hermans D, Deun KV, Martel A, Immerseel FV, Messens W, Heyndrickx M, Haesebrouk F, Pasmans F (2011). Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 42:82.
Crossref
|
|
|
|
Jorgensen F, Bailey R, Williams S, Henderson P, Wareing DRA, Bolton FJ, Frost JA, Ward L, Humphrey T J (2002). Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. Int. J. Food Microbiol. 76:151-164.
Crossref
|
|
|
|
Mohammed HF, Hassan MK, Bakir SS (2004).Campylobacter jejuni gastroenteritis in children in Basrah-Iraq. Mjbu.22(1&2):1-5
|
|
|
|
Nayak AK, Wilson DL, Linz L, Rose JP, Mohanty PK, Das BK (2014). DNA Sequence Analysis of gyrA provides a Rapid and Specific Assay to Identify Arcobacter butzleri Isolates from the Environment. Int. J. Curr. Microbial. Appl. Sci. 3(4):512-529.
|
|
|
|
Petersen L, Nielsen EM, On SL (2001). Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Vet. Microbiol. 82:141-154.
Crossref
|
|
|
|
Saliih DS, Al-Saad MR (1994).Isolation and identification of thermophilic Campylobacters from diarrheal children in Baghdad. J. Islam. Acad. Sci. 7(2):88-92.
|
|
|
|
Schielke A, Rosner BM, Stark K (2014). Epidemiology of Campylobacteriosis in Germany – Insights from 10 years of surveillance. BMC Infect. Dis. 14(30):1-8.
Crossref
|
|
|
|
Schouls LM, Reulen S, Duim B, Wagenaar JA, Willems RJL (2003). Comparative Genotyping of Campylobacter jejuni by Amplified Fragment Length Polymorphism, Multilocus Sequence Typing, and Short Repeat Sequencing: Strain Diversity, Host Range, and Recombination. J. Clin. Microbiol. 41(1):15-26.
Crossref
|
|
|
|
Stoyanchev TT (2004). Detection of Campylobacter using standard culture and PCR of 16SrRNA gene in freshly chilled poultry and poultry products in a slaughterhouse. Trakia J. Sci. 22(3):59-64.
|
|
|
|
Turowski EE, Shen Z, Ducore RM, Parry NMA, Kirega A, Dewhirst FE, Fox JG (2014).Isolation of a Campylobacter lanienae-like Bacterium from Laboratory Chinchillas (Chinchilla laniger). Zoonoses and Public Health 61(8):571-580.
|
|
|
|
Weis AM, Miller WA, Byrne BA, Chouicha N, Boyce WM, Townsend AK (2014). Prevalence and Pathogenic Potential of Campylobacter Isolates from Free-Living, Human-Commensal American Crows. Appl. Environ. Microbiol. 80(5):16391644.
Crossref
|
|
|
|
Woese C R (1987). Bacterial evolution. Microbiol. Rev. 51:21-271.
|
|
|
|
World Health Organization (2012). The global view of Campylobacteriosis: Report of an expert consultation Utrecht, Netherlands. Available at:www.who.int/iris/bitstream/10665/80751/1/978924 1564601_ eng.pdf . [Accessed on 1, January, 2013].
|
|
|
|
Zhang MJ, Qiao B, Xu XB, zhang JZ (2013). Development and application of a real-time polymerase chain reaction method for Campylobacter jejuni detection. World J. Gastroenterol. 19(20):3090-3095.
Crossref
|