The aim of this study was to verify differences in the tolerance to suboptimal temperatures of eight rice mutant genotypes in relation to their cultivars of origin in the initial stages of development. During the germination period, the germination percentage and the germination rate index were evaluated at temperatures of 25 and 13°C. For the vegetative stage enzymatic indicators, photosynthetic pigment concentration and electrolyte leakage were verified. The relative decrease in the germination percentage of mutants QUE 47, QUE 30 and QUE 33 was significantly lower (p ≤ 0.01) than that of cv. BRS Querência. The principal component analysis using the values of the relative decreases of the germination percentage and of the germination rate index confirmed this distinction and the mentioned genotypes were classified into the group with a higher cold tolerance than BRS Querência. The BRS Fronteira genotype did not differ from its mutants for the variables analyzed during germination. In the vegetative stage there were no differences (p ≤ 0.05) between the mutant genotypes and BRS Querência for the analyzed variables. The induced mutation increased the tolerance to suboptimal temperature in the germination period in the genotypes QUE 47, QUE 30 and QUE 33, but not for the vegetative stage.
Rice (Oryza sativa L.) is one of the most important crops in the world and it is essential that its production meet a growing demand (Peyman and Hashem, 2010). Brazil is the ninth largest rice producer, with a production of 12,430,000 tons in the 2014/2015 crop and a total area of 2,280,000 ha, which represents about 2% of world production (Faostat, 2016). The state of Rio Grande do Sul (RS), where flood irrigation production systems prevail, is the main national producer, responsible for 69% of the national production (Conab, 2016).
In Rio Grande do Sul, the occurrence of low temperatures is a major stress factors that limits the increase of grain yield in the crop (Walter et al., 2014). At temperatures below 20°C, rice plants exhibit a wide range of damage caused by cold, depending on the duration of exposure and the stage of development, which can result in a weak establishment of the plant stand, growth retardation and non-uniform maturation (Kim et al., 2012).
Low temperatures do not only generate obvious physical damage to rice plants, but also stimulate physiological fluctuations, such as changes in the concentration and fluorescence of chlorophyll, increased electrolyte leakage (EL) and increased quantities of reactive oxygen species (ROS), malondialdehyde (MDA), sucrose, lipid peroxidation, proline and other metabolites (Zhang et al., 2014). Bekele et al. (2014) claim that reduction in the chlorophyll concentration may indicate the effect of low temperatures in plants, since it could inhibit chlorophyll synthesis and chloroplast formation. The damage caused by the cold is also associated to the increase of EL, since a high concentration of ROS may cause a decrease in fluidity and increased permeability of the membrane (Faget et al., 2013). Virtually all biotic and abiotic stresses trigger a general stress response called oxidative stress, which is induced by overproduction and accumulation of ROS and may damage the cellular components and cause dysfunction (Demidchik, 2014). Plant adaptation to adverse conditions may involve altered synthesis rates, protein repair and increasing endogenous levels of antioxidant enzymes, such as superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.1), glutathione reductase (GR, EC 1.6.4.2), peroxidases (POD, EC 1.11.1.7), catalase (CAT, EC 1.11.1.6), and polyphenol oxidase (PPO, EC 1.14.18.1) (Barbosa et al. , 2014).
According to Cordeiro and Rangel (2011), there are several reasons for the stagnation of rice productivity in Brazil, but the factor that most impairs genetic gain is the limited genetic variability of populations subjected to selection. An important tool to generate desired genetic variations is the induction of mutation with the use of mutagenic agents (Parry et al., 2009). Over the past 75 years, more than 3200 mutant varieties have been registered, as listed in the database of mutant strains of the International Atomic Energy Agency (IAEA), including 817 rice strains, 254 wheat strains and 96 corn strains (Iaea, 2016). The plant improvement strategy based on mutations has involved the enhancing of the adapted cvs., by altering one or two important traits of agronomic interest, such as early flowering, grain yield and resistance to disease, salinity and low and high temperatures (Mba, 2013)
Silva (2013) reported that mutation induction by irradiation can positively affect different physiological and biochemical processes of plants. However, it may also lead to the manifestation of undesirable characteristics, which result in the reduction of production potential and whose expression depends on the environment. Therefore, the submission of mutant genotypes to the stressful environment and analysis of their behavior is of great relevance. Thus, considering that stress caused by the cold is a significant problem for rice production in temperate and tropical regions at high altitudes (Kim et al., 2012), the aim of this study was to evaluate the differential tolerance to low temperatures during the germination and vegetative stages of eight rice mutants genotypes with high productive potential in relation to their cvs. of origin (BRS Querência and BRS Fronteira).
Eight third generation mutant genotypes of rice (QUE 07, QUE 30, QUE 33, QUE 47, FRO 01, FRO 09, FRO 57 and FRO 58) and their cvs. of origin (BRS Querência and BRS Fronteira), belonging to the working collection from Embrapa Clima Temperado were used in this study. Mutant genotypes were obtained by ionizing radiation with Cobalto60 in doses ranging from 200 to 350 Gy applied to seed of cvs. BRS Querência and BRS Fronteira. The choice of these genotypes was based on characters of agronomic interest that are superior to those of their cvs. of origin, previously assessed by Silva et al. (2011) and Silva (2013), such as cycle reduction and reduction in the number of sterile spikelets and the height of plants, as well as increased amount of grains per panicle and 1000-grain weight. The seeds were harvested in 2012/2013 and selected based on size uniformity and absence of spots.
Experiment 1: Germination at low temperatures
Seeds of the eight mutant genotypes and their cvs. of origin were placed in germ test paper rolls moistened with an amount of water distilled equivalent to 2.5 the paper weight, following the criteria established by the Guidelines for Seed Analysis (Regras para Análise de Sementes; Brasil, 2009). In each roll of paper, 100 seeds were placed, with a total of 400 seeds per genotype per treatment. Then, these rolls were placed in plastic bags and incubated in germination chambers with a photoperiod of 12 h under two constant temperatures, 25°C (ideal) and 13°C (suboptimal), where they remained for 14 days, with replenishment of water as necessary.
The variables evaluated were: Germination percentage (GP), germination rate index (GRI), according to the equation proposed by Maguire (1962) and length of the aerial part (LAP) and the root system (LR), evaluating 10 plants per replicate at 14 days after sowing (DAS). The seeds with radicles of at least 2 mm in length were considered germinated. The relative decrease of GP was calculated by the equation:
RDGP (%) = (GP25×GP13)/GP25 × 100 Where RDGP is the relative decrease in the germination percentage, GP25 is the germination percentage at 25°C and GP13 the germination percentage at 13°C.
The relative decrease of GRI was calculated by the equation: RDGRI (%) = (GRI25×GRI13)/GRI25 ×100
Where RDGRI is the relative decrease of the germination rate index, GRI25 is the germination rate index at 25°C and GRI13 is the germination rate index at 13°C. A completely randomized design in a 10 × 2 factorial scheme was used (10 genotypes, 2 temperatures), with four replicates represented by 100 seeds.
Experiment 2: Low temperature conditions in a greenhouse in the early stages of development
Based on the results obtained in experiment 1, the following mutant genotypes were selected: QUE 07, QUE 30, QUE 33, QUE 47 and their cv. of origin, BRS Querência. The seeds were sown in polypropylene vases with a capacity of 250 mL, filled with organic compost recommended for the cultivation of rice, at a depth of 0.5 cm. The vases were kept in the greenhouse and irrigation was carried out daily with distilled water. When the plants reached the V3 stage (Counce et al., 2000), fully expanded leaf segments were collected and frozen in liquid nitrogen and stored in ultrafreezer at -80°C.The experiment was conducted in a greenhouse in the period from 14 October to 11 November 2015. According to the Agrometeorological Station of Pelotas, located 1 km from the experimental area, at latitude 31°52'00"S, longitude 52°21'24"W and 13.24 m altitude, during this period the average temperature was 17.9°C, with an average minimum of 14.9°C, absolute minimum of 5.8°C, average maximum of 21.6°C and absolute maximum of 28.6°C. During the period, there was a significant influence of the “El Niño” effect, with cooler temperatures than typical in the Southern region of Brazil.
The variables analyzed were chlorophyll concentration (a, b and total), carotenoid concentration and antioxidant activity of enzymes SOD, CAT, APX and GR. The method of Arnon (1949) was used for determination of photosynthetic pigments. In a darkroom, 75 mg of leaf tissue were macerated in 10 mL of 80% acetone, the suspension was filtered and absorbance was read at 470, 645 and 663 nm wavelengths, using a spectrophotometer (Ultrospec 9000, GE). Pigment concentrations were determined by the equations of Hendry and Price (1993). The method of Azevedo et al. (1998) was used for protein extraction (1998), where 200 mg of leaves were macerated in liquid nitrogen plus 20% polyvinylpyrrolidone and diluted in 2 mL of extraction buffer, composed of 100 mM potassium phosphate pH 7.8, 0.1 mM EDTA and 10 mM of ascorbic acid. The mixture was centrifuged in microtubes of 2 mL capacity at 13,000 g for 10 min at 4°C, and the supernatant was collected and transferred to a new microtube with a 1.5 mL capacity, stored at -20°C until the completion of the analyses.
The total protein concentration of the samples was quantified using the method of Bradford (1976), with bovine serum albumin (BSA) as standard. SOD activity was assessed for its capacity to photoreduce nitrotetrazolium blue (NTB), as described by Giannopolitis and Ries (1997). One unit of SOD corresponds to the amount of enzyme able to inhibit NTB photo reduction by 50% under assay conditions. The composition of the reaction medium was 50 mM potassium phosphate pH 7.8, 14 mM methionine, 0.1 µM EDTA, 75 µM NTB and 2 µM of Riboflavin. CAT activity was determined as described by Azevedo et al. (1998), monitored at 240 nm in a spectrophotometer for degradation of H2O2 in a reaction medium with 100 mM potassium phosphate pH 7.0 and 12.5 mM H2O2. APX activity was determined according to the method described by Nakano and Asada (1981), by monitoring the rate of ascorbate oxidation at 290 nm in a reaction medium with 100 mM potassium phosphate pH 7.0, 0.5 mM ascorbic acid and 0.1 mM H2O2. GR activity was evaluated according to Cakmak et al. (1993) by monitoring the rate of NADPH oxidation at 340 nm for the decrease in absorbance in a reaction medium with 50 mM potassium phosphate pH 7.8, 1 mM of oxidized glutathione and 75 µM NADPH.
The experimental design was completely randomized, using five genotypes with six replicates each with six plants.
Experiment 3: Controlled low temperature conditions in the seedling stage
Seeds of mutant genotypes QUE 07, QUE 30, QUE 33, QUE 47 and their cv. of origin, BRS Querência were sown in polypropylene vases with a capacity of 250 mL filled with washed sand at a depth of 0.5 cm. The vases were kept in a BOD type growth chamber with a controlled temperature of 25 and 0.2°C and photosynthetic photon flux density (PPFD) of 26 µmol m-2 s-1 and photoperiod of 16 h. Irrigation was carried out daily alternating between the use of 20 mL of distilled water and an equal volume of 0.5 nutrient solution described by Hoagland and Arnon (1950) and the use of triple washing of sand by percolation of distilled water before the addition of nutrient solution in order to avoid the accumulation of ions. When the seedlings reached the V4 growth stage (Counce et al., 2000), the low temperature treatment was initiated, where the plants remained in the same conditions as above, except the temperature, modified to 13°C.
The response of rice plants to low-temperature stress was evaluated through quantification of chlorophyll and carotenoid concentrations, EL and activity of antioxidant enzymes associated with stress response in plants, SOD, CAT and APX.
The variables were examined for five times of exposure: 0, 24, 48, 72 and 96 h. Fully expanded leaf segments were used as plant material and those intended for evaluation of enzyme activity and quantification of photosynthetic pigments were frozen in liquid nitrogen and stored in ultrafreezer at 80°C.
The extraction and quantification of proteins and pigments and the analysis of enzyme activity were carried out according to the respective methods described in experiment 2.
EL measurement was based on the method described by Kim et al. (2012). Six sections of three centimeters in length of fully expanded leaves were collected and washed with distilled water. The samples were placed in assay tubes of 10 mL capacity containing 5 mL of distilled water and incubated for 24 h at room temperature, at which time the first value for electrical conductivity (E1) was measure using a portable CD-30 digital conductivity meter (Corning). The samples were autoclaved for 20 min at 120°C and after reaching room temperature the second conductivity value (E2) was measured.
EL was expressed as: EL (%) = (E1/E2) × 100.
The experimental design was completely randomized in a 5 × 5 factorial (5 genotypes 5 times of exposure to cold), with three replicates of six plants per treatment.
Statistical analysis
The data from experiment 1 were submitted to analysis of variance and means were compared by Tukey test at 1% probability of error. The mean value for each genotype was calculated by principal component analysis (PCA), using the MINITAB statistical software 17 (Minitab). The low temperature tolerance of each genotype was determined according to the results for means and PCA comparing the mutant genotypes to their respective cvs. of origin. For experiments 2 and 3, the data were subjected to analysis of variance and means were compared by Tukey test at 5% probability of error using the Winstat 2.0 statistical software (Machado and Conceição, 2002).
Analysis of means showed that the mutant genotypes from the cv. BRS Querência presented no decrease (p ≤0.01) of GP under low-temperature conditions when compared to favorable germinating conditions (Table 1). However, for the cv. BRS Querência there was a significant decrease. When comparing values of GP between the genotypes at 13°C, QUE 47, QUE 30 and QUE 33 presented values of 150, 137 and 108%, higher than that of Querência BRS, respectively.
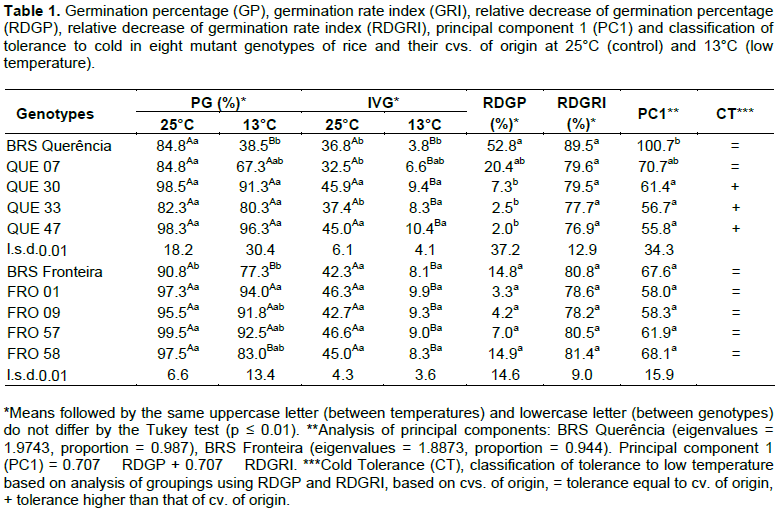
Considering the existence of variation in GRI and GP, whether significant or not, between the genotypes at 25°C, the relative decreases between ideal and suboptimal conditions were compared for these variables. The relative decrease in the germination percentage (RDGP) for QUE 47, QUE 30 and QUE 33 was significantly lower than that found for BRS Querência. Based on principal component analysis using the RDGP and RDGRI values (Figure 1 and Table 1), the value calculated from the first component of these mutants differed (p ≤ 0.01) in a similar mode for RDGP. Thus, QUE 47, QUE 30 and QUE 33 were classified as genotypes with higher tolerance (+) than BRS Querência, while QUE 07 did not differ (=) from BRS Querência (Table 1).
In a separate analysis of GP in BRS Fronteira and its mutants, the cv. of origin differed from the mutant FRO
01 at 13°C. However, this result cannot be attributed to the effect of suboptimal temperature, since this difference also occurred at the optimal germination temperature. Through the RDGP and RDGRI, the effect of the cold was analyzed separately and there were no differences between mutants and the cv. Of origin. Based on principal component analysis and means, the mutants from the cv. BRS Fronteira were grouped with the genotype of origin (=) for tolerance to suboptimal temperature in the germination period. There was no significant difference between the mutant genotypes and their cv. of origin for LAP and LR at a fixed temperature. However, in the comparison between temperatures, all genotypes differed significantly (data not presented).
After submitting the rice seeds to the low temperature environment, this study confirmed the hypothesis of maintenance and even increase of cold tolerance in the germination stage of mutant genotypes in relation to the cv. of origin BRS Querência, which already offers a good level of cold tolerance in the stages of germination and emergence when compared to other genotypes grown in the irrigated area most affected by cold in Rio Grande do Sul (Fagundes et al., 2010). In regard to the cv. BRS Fronteira, there was no difference in tolerance to cold in the germination period when compared to its mutant genotypes.
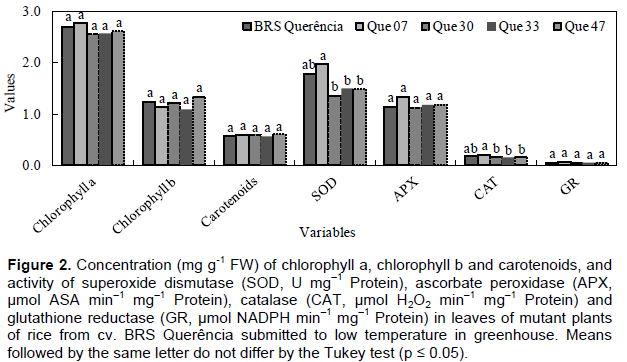
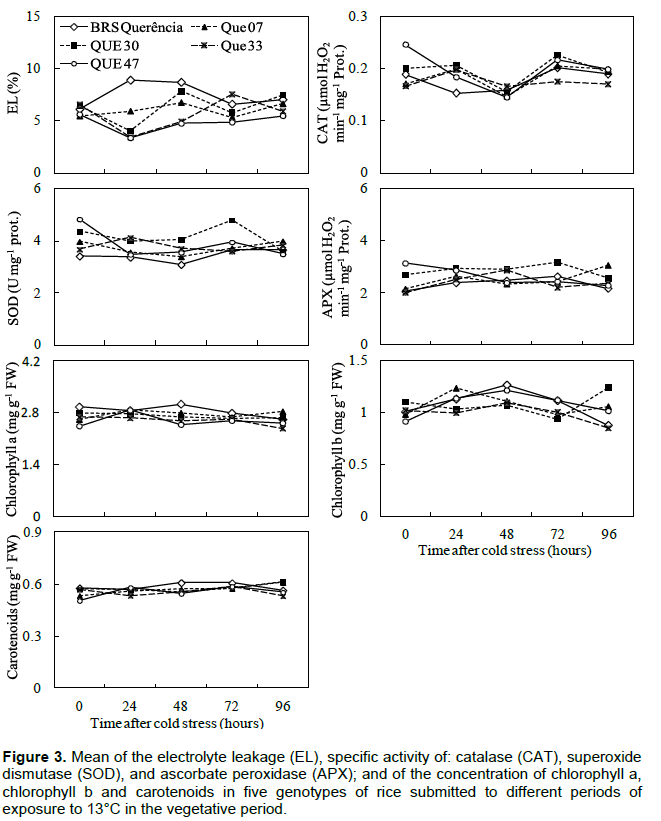
In the experiments carried out in the vegetative period, no changes were observed in the variables indicative of tolerance to low temperatures (antioxidant enzyme activity, EL and concentration of photosynthetic pigments) for mutant genotypes compared to cv. Of origin, BRS Querência (Figures 2 and 3). Bonnecarre et al. (2011) confirm the importance of these variables to evaluate the tolerance to low temperatures of rice at the vegetative stage. Aghaee et al. (2011) also showed a differential reduction of chlorophyll concentration between contrasting genotypes regarding cold tolerance when they were submitted to low temperature conditions, a fact not observed in the present study (Figures 2 and 3). In the comparison between the mutant genotypes in experiment 2, QUE 07 presented the greatest SOD and CAT activity (Figure 2). The similar behavior of SOD and CAT is not surprising since CAT activity is more related to the removal of excess H2O2 than APX in response to certain stresses, Li et al. (1981), analyzing the performance of rice genotypes in different stages of growth, concluded that the differential tolerance to low temperatures does not occur only between distinct genotypes, but also between the phases of growth within each genotype. The results obtained in this study demonstrate the existence of mutant genotypes with greater cold tolerance in the germination period and the lack of difference in tolerance in the vegetative period in relation to the cv. of origin. This may be due to different genes for tolerance and different physiological mechanisms involved in tolerance to the cold at each stage of growth (Cruz and Milach, 2000). The differential behavior of these mutants is probably related to genetic changes acquired by irradiation of seeds and the results of this study demonstrate maintenance of these characteristics, even after three generations.