ABSTRACT
In Olive (Olea europaea L.) a critical balance of concentration and time was necessary to achieve high germination percentages without loss of viability of the seeds. Therefore olive cvs. Coratina and Pendolino seeds were subjected to chemical treatment with HCl, GA3, NaOH and water for two dips time (12 and 24 h) to determine the most appropriate treatment to improve seed germination and to reduce seed germination time. Among studied treatments (GA3 500 ppm for 12 h dip, GA3 500 ppm for 24 h dip, HCl 1N for 12 h dip, HCl 1 N for 24 h dip, NaOH 1 N for 12 h dip, NaOH 1 N for 24 h dip, Water 12 h, Water 24 h, Control) tested, the best results were recorded in both cultivars with GA3 500 ppm for 12 h dip as compared to control in both the cultivars suggesting that this treatment could be most the effective to enhance of seed germination and minimize the seed germination time.
Key words: Seed germination, scarification, olive.
Olive (Olea europaea) is undoubtedly one of the world’s oldest cultivated crop originated from Asia Minor, it has tremendous potential in India especially in mid hills and warm temperate regions of North Western Himalaya. It is a crop of Mediterranean region but grows well even under mild temperate conditions if chilling requirements are met. Olive can be propagated by seed, cutting, grafting and suckers, but most common method is the rooting of stem cuttings under mist system. Stem cuttings of selected cultivars are hard to root (Fabbri et al., 2004). The efficiency of propagation is low and the root systems of rooted cuttings are also shallow spreading to 0.9 to 1.2 meter even in deep soils (Bandino et al., 1999). Growing seedlings and grafting the selected cultivars on them may be suitable alternative for olive propagation. Olive seed germination is erratic, very slow and proceeds for 2 to 3 years (Sotomayor-Leon and Caballero, 1990; Zuccherelli and Zuccherelli, 2002) and germination percentage might not exceed 10% in many cultivars (Acebcdo et al., 1997). The major barrier for olive seed germination is the stony endocarp in addition to other causes of dormancy including seed coat, endosperm and embryo itself (Lagarda et al., 1983a; Lagarda and Martin, 1983; Prista et al., 1999 and Lagarda et al., 1983b). Seed germination of 'Manzanillo olive was improved by using stoneless seeds (Crisosto and Sutter, 1985). However, low germination percentage was obtained with other cultivars using stonless seeds (Acebedo et al., 1997). It was reported that 28% of olive seed dormancy is imposed by the endocarp and 56% by the endosperm (Sotomayor- Leon and Caballero, 1994). For commercial olive seed germination, breaking olive endocarps described by Sotomayor-Leon and Caballero (1990) does not always work well. Chemical scarification has been widely used to overcome physical seed dormancy (Hartmann and Kester, 2002). Germination percentage of three olive cultivars was improved after the stony seeds were scarified with 0.1 N NaOH and H2SO4 at 0.1 N or 1 N concentrations (Bandino et al., 1999). Chemical agents such as norflurazon and continuous washing in running water have also been used to overcome olive seed dormancy (Sotomayor- Leon and Altisent, 1994). The aim of this work was to determine the effect of different treatments on the germination of Coratina and Pendolino olive cultivars seeds.
Fruits from ' olive cv. Coratina and Pendolino from olive research block at the Central Institute of temperate Horticulture, Srinagar were harvested in October, 2010 and 2011 when the color was changed from yellow green to violet. Fruit flesh was separated by soaking them for 15 min in 4% NaOH. The stony seeds were cleaned with sand and water, and were then soaked in running water for 12 h to remove residues. Seed were immediately sown with the following treatments like GA3 500 ppm for 12 h dip, GA3 500 ppm for 24 h dip, HCl 1 N for 12 h dip, HCl 1 N for 24 h dip, NaOH 1N for 12 h dip, NaOH 1 N for 24 h dip, Water 12 h, Water 24 h, Control (without any soaking treatment). Fifty seeds were used in each treatment with five replicates for each cultivar as per methods described by Gomez and Gomez (1984). The stony seeds in the 9 treatments were left to germinate in plates filled with sand containing 90% moisture and placed in a mist chamber at 20 to 25°C. Germination time is calculated from sowing to emergence of the hypocotyle and germination percentage was determined by the emergence of the number of hypocotyls. Statistical analysis was done using SAS software package (SAS Inst. 2012), for mean differences the analysis of variance was followed by DMRT test at 1% probability level.
Both average times taken for germination and average germination percentage were influenced by cultivars and treatments; however, treatments did not always produce the same effects in the two cultivars. In Coratina cultivar stony seeds soaked in GA3 500 ppm for 12 h dips was found most effective (Figure 1) and statistically significant in terms of early germination (Figure 2) of stony seeds with a maximum germination percentage. This increase in seeds germination percentage might be related to the initial enzyme induction and to the activation of reserve food – mobilizing systems by Gibberellins which have also been used to enhance germination and stimulate early seedling emergence and growth (Hopkins and Hüner, 2004). Hopkins and Hüner (2004) stated that gibberellins prominently involved in seed germination and mobilization of endosperm reserves during early embryo growth as well as flower and fruit development.
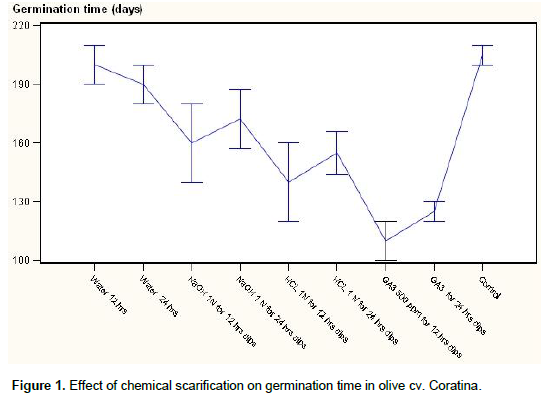
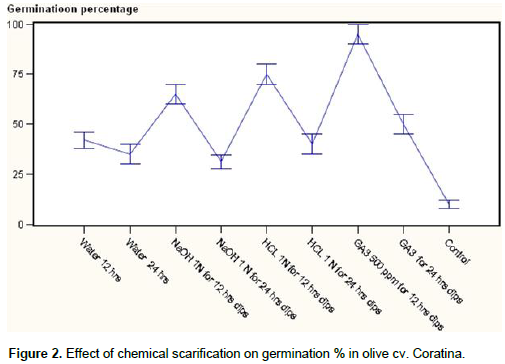
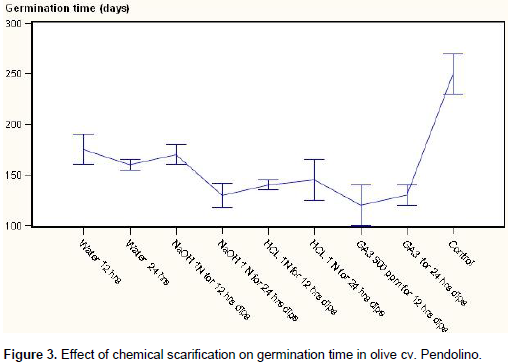
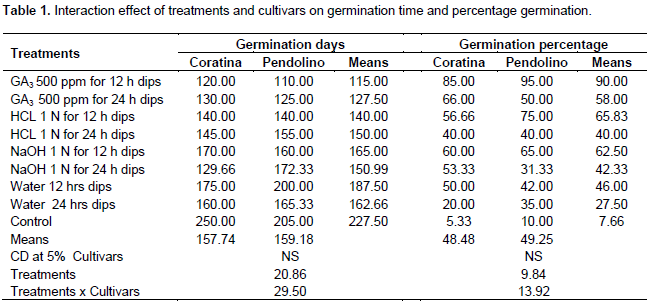
However as the seeds soaking time increased the germination percentage was decreased. Treatments NaOH 1 N for 24 h dips, GA3 for 24 h dips also found at par with the best treatments for seed germination and found similar at significant level. In Pendolino cultivar also minimum (110.00) time taken for germination (Figure 3) of stony seeds was recorded with the treatment of GA3 12 hrs as compared to control (205 days) showing in the same time the highest germination (Figure 4) percentage (95%). Second best treatment was found HCl 1N for 12 h dips however time taken for germination was little higher than GA3 for 24 h dipped. Pendolino seeds germinated faster than Coratina cultivars, with an average germination time of 110 days as compared to 250 days for Coratina. The acid and base treatment was found not as effective as GA3 in increasing germination percentage and reducing the germination time that might be due to the their corrosive effect which may cause damages to the embryo or the treatment was not as effective as GA3. In treatment cultivar interaction (Table 1) the cultivars did not differ significantly however among the treatments, minimum germination time (115 days) and maximum seed germination percentage (90%) were recorded with GA3 500 ppm for 12 h dips and significantly differ as compared to control that showed a germination time of 227.5 days and a germination rate of 7.65%.

Thus, the increased percentages of germination obtained when olive seeds are treated with GA3 soaking overnight would improve the feasibility of developing breeding programs for olives and would enable nurserymen to grow olives from seed without serious loss.
The author(s) have not declared any conflict of interests.
REFERENCES
Acebedo MM, Laver S, Linnan J, Troncoso A (1997). In vitro germination of embryos for speeding up seedling development in olive breeding programs. Scienta Hortic. 69:207-215.
Crossref |
|
|
Bandino G, Sedda P, Mulas M (1999). Germination of olive seeds as affected by chemical scarification, hot water dip and gibberellic acid treatments. Acta Hortic. 474:35-38.
Crossref |
|
|
|
Crisosto C, Sutter E (1985). Role of endocarp in 'manzanillo' olive seed germination. J. Am. Soc. Hortic. Sci. 110:50-52. |
|
|
|
Fabbri A, Bartolini G, Lambardi M, Kailis S (2004). Olive propagation manual, Landlinks Press, Collingwood, 141. |
|
|
|
Gomez KA, Gomez AA (1984). Statistical Procedures for Agricultural Research, 2nd Edn., John Wiley and Sons Inc., New York. |
|
|
|
Hartmann HT, Kesler DE (2002). Plant Propagation. 7th Ed. Prentice-Hall. Inc. New Jersey. |
|
|
|
Hopkins WG, Hüner NPA (2004). Introduction of plant physiology. 3rd Edition. John Wiely & Sons, Inc. USA. |
|
|
|
Lagarda A, Martin G (1983). 'Manzanillo' Olive seed dormancy as influenced by exogenous hormone application and endogenous adscisic acid concentration. Hortsciences 18(6):869-871. |
|
|
|
Lagarda A, Martin GC, Kester DE (1983a). Influence of Environment, Seed Tissue, and Maturity on 'Manzanillo' Olive Seed Germination. Hortscience. 18(6):868-869. |
|
|
|
Lagarda A, Martin GC, Polito VS (1983b). Anatomical and morphological development of 'manzanillo' olive seed in relation to germination. Am. Soc. Hortic. Sci. 108:868-869. |
|
|
|
Prista T, Voyiatzi C, Metaxas D, Voyiatzis D, Koutsika – Sotiriou M (1999). Observation on the germination capacity and breeding value of seedlings of some olive cultivars. Acta Hortic. 474:117-120. |
|
|
|
SAS Institute (2012). SAS enterprise guide, Version 9.2. SAS Inst., Cary, NC, USA. |
|
|
Sotomayor-Leon EM, Caballero JM (1990). An Easy Method of Breaking Olive Stone to Remove Mechanical Dormancy. Acta Hortic. 286:113-116.
Crossref |
|
|
|
Sotomayor-Leon EM, Caballero JM (1994). Propagation of 'Gardal Sevillno' Olive By Grafting Onto Rooted Cuttings or Seedlings under Plastic Closed Frame without Mist. Acta Hortic. 356:39-42. |
|
|
|
Sotomayor-Leon EM, Altisent JMD (1994). Breaking of dormancy in olive (Olea Europaea L.) seeds. Acta Hortic. 365:137-142. |
|
|
Zuccherelli G, Zuccherelli S (2002). In vitro propagation of fifty olive cultivars. Acta Hortic.586:931-934.
Crossref |