Mungbean, Vigna radiata (L.) Wilczek commonly known as green gram is an important legume crop widely grown in many Asian countries including India, Bangladesh, Bhutan, China, Myanmar, Nepal, Sri Lanka, Thailand and Pakistan. In India, it occupies third place after chickpea and pigeonpea (Ved Ram et al., 2008). Mungbean crop grow once in a year, except some regions of the country where it is grown in summer season for fodder as well as grain purpose. Due to its more luxurious growth and more vegetative canopy, numbers of insect pests attack from seedling to maturity stage which is detrimental factor for their production and cause severe losses in their productivity. Since it is grown in tropical climates, bunches of insect pests play an important role in marketable production. The magnitude of insect pest losses to mungbean has been estimated by Panchabhavi and Khadam (1990); Rao et al. (1990) and Sharma et al. (1991). Most of these insects are polyphagous and feed on a wide range of variety of legumes and non-legumes. Mungbean is attacked by different species of insect pests but sucking insect pests (aphid, jassids, leaf hopper and whitefly) are of the major importance (Biswass et al., 2008). Among the various constraints, yellow mosaic virus disease caused by yellow mosaic Gemini virus is one of the major factor in the cultivation of mungbean, particularly, in northern states. The vector of this disease is whitefly (Bemisia tabaci Genn.). Sastry and Singh (1973) and Khattak et al. (2004) have observed it as major importance and that even low population of whitefly is capable for wide transmission of yellow mosaic virus. B. tabaci is common in India and cause direct damage by sucking the cell sap (Sehgal and Ujagir, 1987). Whitefly sucks the cell sap from the leaves and in case of severe damage, it secretes honeydew which causes sooty mould and hampers the photosynthetic activity. For control of vector transmitted viral diseases, controlling the insect vectors using chemical insecticides has been the common practice among the farmers. Previously many research workers have evaluated synthetic chemicals against sucking pests of mungbean (Gopal and Srivastava 1997). A drastic reduction in the incidence of MYMV was recorded where whitefly population was reasonably controlled by using chemical insecticides. But indiscriminate use of pesticides resulted in the development of resistance in the target pest species, resurgence of whitefly and environmental pollution. Contrastingly, neem products like neem seed kernel extract (NSKE), 5% was found effective in managing the population of whitefly in mungbean (Hussain et al., 2001). In order to arrest the mungbean damage due to these insects, the insecticides are mainly relied upon to keep the pest population below the economic threshold level (Chhabra and Kooner, 1985). Keeping these facts in view present study was conducted on mungbean to find out the bioefficacy of some insecticides and biorationals against whitefly in order to find an effective and economic control of this pest under agro-ecological conditions of Haryana state.
Experimental trials were conducted during kharif season 2010 to 2011 and 2011 to 2012 at Pulses Research Farm, Department of Genetics and Plant Breeding, CCS Haryana Agricultural University, Hisar. Mungbean cultivar ‘Asha’ (20 kg ha-1) was sown as a test variety, at 30 × 10 cm spacing in a randomized block design with three replications. For seed treatment, 60 and 100 ml quantity of imidacloprid and dimethoate was mixed in water and made a total volume of emulsion 300 ml. Seed treatment was done, one day before sowing in the evening. The treated seeds were spread on the polythene sheet and left overnight for drying. Sowing was done in the morning hours next day. The crop was raised under recommended agronomical package of practices, except the plant protection measures (Anonymous, 2004). Monitoring was done regularly for recording the incidence of whitefly. When the adult whitefly population reached three adults per plant, treatments were imposed to manage whitefly population. The adult whitefly population per cage per plant was recorded with the help of split cage (Nath, 1994). Observations on adult whitefly population were recorded 30 days after sowing and one, three, seven, ten and fourteen days after spray. The observation on the total number of plants and the yellow mosaic virus infested plants per plot were also recorded at the same period as for whitefly population and percent plant infection was worked out for each plot. The percent avoidable yield loss was also calculated with the help of following formula given by Sharma et al. (1991):

The grain yield obtained and cost of plant protection measures, including labour charges and spraying charges and net profit per hectare was also calculated. Cost Benefit Ratio (ICBR) was worked out as the net profit to the cost of plant protection.
Seeds were treated with Imidacloprid 200 SL at 3 ml/kg seed and dimethoate 30 EC at 5 ml/kg seed. These treatments were compared with seed treatments of these insecticides followed by spraying of triazophos 40 EC at 0.04 and 0.02% and NSKE 5% at 30 days after sowing. Nine treatments viz., (T1) seed treatment with Imidacloprid 3 ml/kg, (T2) seed treatment with dimethoate 5 ml/kg, (T3) T1 + triazophos 0.04%, (T4) T1 + triazophos 0.02%, (T5) T2 + triazophos 0.04%, (T6) T2 + triazophos 0.02%, (T7) T1 + NSKE 5%, (T8) T2 + NSKE 5% and (T9) untreated control were evaluated against whitefly population. Data was subjected to analysis of variance given by Gomez and Gomez (1984).
Bioefficacy of insecticides and biorationals for management of whiteflies
Data recorded in kharif 2010 to 2011 presented in Table 1 revealed that at 30 days after sowing whitefly has reached the ETL level and minimum population of whitefly was in plots where seeds treated with dimethoate 30 EC at 5 ml/kg seed and it ranged from 2.4 to 3.5 adults/ plant. The initial 30 days of the crop is crucial for the well establishment of the seedlings. So, the lower whitefly populations were harboured in those plots, where seeds were treated with dimethoate. Three days after spraying, the lower population of whitefly (1.6 adults/ cage/ plant) was recorded in treatment T8 (seed treated with dimethoate followed by NSKE 5% spray) and it was statistically at par with treatment T7, seed treatment with imidacloprid followed by NSKE 5% spray (2.0 adults/ cage/ plant), T2, seed treatment with dimethoate (2.4 adults/ cage/ plant), T3, seed treatment with imidacloprid followed by triazophos 0.04% (2.3 adults/ cage/ plant) and T5 seed treatment with dimethoate followed by triazophos 0.04% (2.7 adults/ cage/ plant). The higher population of whitefly (4.2 adults/ cage/ plant) was recorded in treatment T1 (seed treatment with imidacloprid 3 ml/kg) which was statistically at par with untreated control.
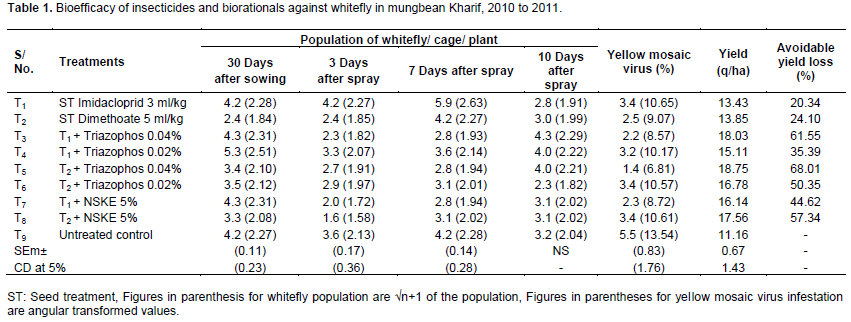
Observations recorded 7 days after spray, minimum population of whitefly was recorded in treatments T3 and T5 (2.8 adults/ cage/ plant) where triazophos 40EC at 0.04% was sprayed after seed treatment and these werestatistically at par with treatment T4 (T1 + triazophos 0.02% spray) 3.6 adults/ cage/ plant, T6 (T2 + triazophos 0.02% spray) 3.1 adults/ cage/ plant, T7 (T1 + NSKE 5% spray) 2.9 adults/ cage/ plant and T8 (T2 + NSKE 5% spray) 3.1 adults/ cage/ plant. The similar findings were reported by Khan et al. (2012), who reported that minimum whitefly population was observed when mungbean seeds were treated with imidacloprid. The results differed from those of Singh and Chourasiya (2013), who reported that whitefly population/ cage was maximum in imidacloprid treated blackgram plots as compared to other treatments at three and seven days after spraying.
Data presented in Table 2, regarding mean adult whitefly incidence during kharif 2011 to 2012 revealed that all the treatments were superior over untreated control at 30 days after sowing. The minimum population of adult whitefly (6.0 adults/ cage/ plant) was recorded in treatment T6, seed treatment with dimethoate 5 ml/kg seed. However, maximum population of adult whitefly was found in untreated control plots (12.6 adults/ cage/ plant).
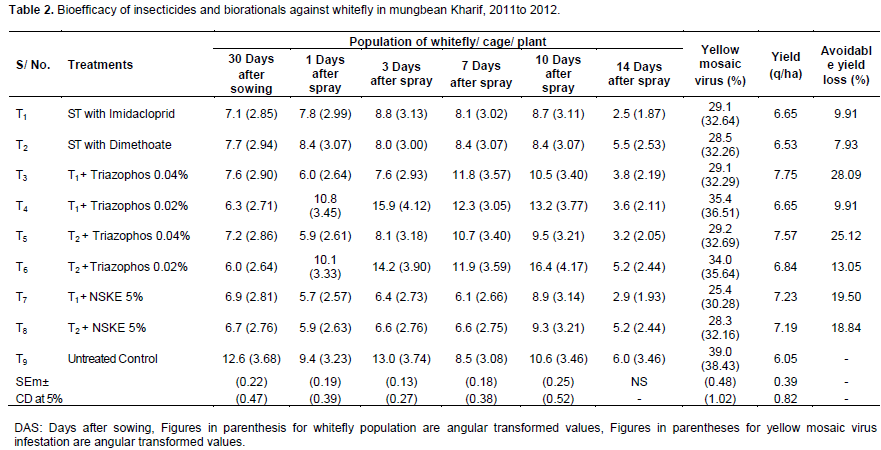
Observations recorded 1 days after spray application revealed that treatment T7, seed treatment with imidacloprid followed by spray of NSKE 5% and treatment T8, seed treatment with dimethoate followed by spray of NSKE 5% are equally effective in reducing the adult whitefly population, registering 5.7 and 5.9 adults/ cage/ plant. The highest population of adult whitefly (10.1 and 10.8 adults/ cage/ plant) was observed in treatments T6 and T4, seed treatment with dimethoate followed by spray of triazophos 0.02% and seed treatment with imidacloprid followed by spray of triazophos 0.02%. Similar trends of adult whitefly population. were observed in treatments T7 and T8 after 3rd and 7th days of application of insecticide and recorded 6.4, 6.1 and 6.6 adults/ cage/ plant respectively. The higher population of adult whitefly (14.2, 11.9 and 15.9, 12.3 adults/ cage/ plant) was recorded in treatments T6 and T4, respectively, where lower dosage of triazophos was sprayed. From the above findings it is clear that seed treatment with imidacloprid 3 ml/kg seed, seed treatment with dimethoate 5 ml/kg seed and spray of NSKE 5% was effective up to 7 days after spray application. Spraying of triazophos at 0.02% increased the whitefly population.
Ten days after spray, the lower adult whitefly incidence (8.4 and 8.7 adults/ cage/ plant) was recorded in those plots whose seeds were treated with dimethoate 5 ml/kg and imidacloprid 3 ml/kg seed, respectively. Higher adult whitefly population (16.4 and 13.2 adults/ cage/ plant) was recorded treatments T6, seed treatments with dimethoate followed by spray of triazophos 0.02% and T4, in seed treatment with imidacloprid followed by spay of triazophos 0.02%. Whitefly adult population increased after 10 days of spray of triazophos at 0.02 and 0.04% and spray of NSKE 5% indicating that after 10 days, triazophosand NSKE could not protect the crop and resurgence in whitefly population was recorded. The results are in conformity by Panghal et al. (2008), who reported that seed treatment with imidacloprid, dimethoate and spray of NSKE 5% were the most effective against whitefly in keeping the population low up to 7 days. Masood et al. (2004) observed that seed treatment with imidacloprid 75.03 and Mustafa (2000) 72.76% reduction in whitefly population. Iqbal et al. (2013) also found that imidacloprid was most effective and resulted in a minimum population of whitefly.
The present findings can be compared with those of Afzal et al. (2002) who reported that imidacloprid 25WP at 200 g/acre was found to be most effective for whitefly. Mohan and Katiyar (2000) stated that imidacloprid was the most effective in suppressing the whitefly population and its continuous use resulted in increased whitefly population.
Effect of insecticides and biorationals on yellow mosaic virus infection (%)
The data presented in Table 1, revealed that the minimum yellow mosaic virus infection (1.4%) was recorded in treatment T5 (seed treated with dimethoate followed by spray of triazophos 0.04%) and it was statistically at par with T3, seed treatment with imidacloprid followed by triazophos 0.04%. The highest yellow mosaic virus infection (3.4%) was recorded in treatment T1 (seed treatment with imidacloprid 3 ml/kg), T6 (T2 + spray of triazophos 0.02%) and T8 (T2 + spray of NSKE 5%). The results are differed from Khan et al. (2012) who reported that imidacloprid was most effective to control mungbean yellow mosaic virus. These findings were in agreement with those of Oetting and Anderson (1991) who reported that application of imidacloprid reduces the yellow mosaic virus in greenhouse grown poinsettias as compared to other treatments.
Observations recorded on yellow mosaic virus presented in Table 2 revealed that minimum yellow mosaic virus infection (25.4%) registered in treatment T7, seed treatment with imidacloprid followed by spray of NSKE 5% it was followed by T8, seed treatment with dimethoate followed by spray of NSKE 5% (28.3%). Seed treatment with imidacloprid/ dimethoate and spraying of triazophos at 0.02% could not help in reducing yellow mosaic virus infection and 35.4 and 34.0% MYMV in treatment T4 and T6. These results indicating that the spraying of NSKE 5% showed long time effect against whitefly as compared to untreated control. The present findings on percent yellow mosaic virus infection are in agreement with those of Panghal et al. (2008), who reported that minimum plant infection by yellow mosaic virus was observed in seed treatment with imidacloprid/ dimethoate and NSKE 5% sprayed plots. The similar results are reported by Khan et al. (2012), who reported that chemical imidacloprid was most effective to control mungbean yellow mosaic virus. Earlier, reduction in the infection of yellow mosaic virus by spraying of NSKE (Sethuraman et al., 2001) has also been recorded.
Effect of different treatments on the yield of mungbean and cost benefit ratio
The data on seed yield, net monetary returns and cost benefit ratio are presented in Table 3. The data on seed yield showed that the seed yield in all the insecticides and biorational treatments was significantly higher than the untreated control. However, the highest grain yield of mungbean (13.16 q/ha) was realized in treatment T5, seed treatment with dimethoate followed by spray of triazophos 0.04%. It was followed by treatment T3, seed treatment with imidacloprid + spray of triazophos 0.04% (12.89 q/ha) and treatment T8, seed treatment with dimethoate + spray of NSKE 5% (12.37 q/ha). The lowest yield 8.60 q/ha was registered in untreated control plots, indicating immense damage potential of whitefly on mungbean.
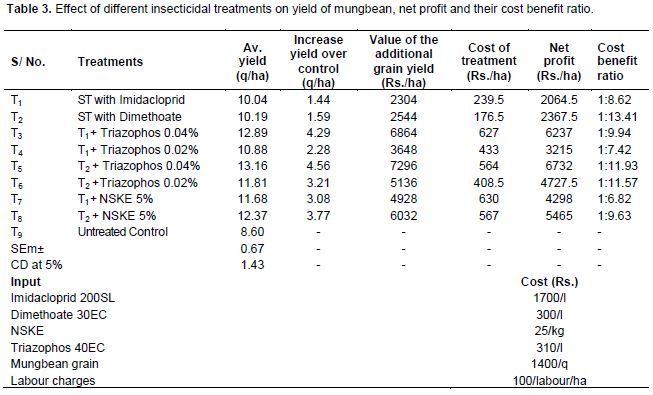
The contrasting results are observed by Shah et al. (2007), who reported that the highest seed yield of 1563 kg ha-1 was recorded from the plots where imidacloprid was applied to control sucking insect pests of mungbean. These results are in conformity with those of Ujagir and Chaudhry (1997), Ahmad et al. (1998) and Deka et al. (1998). They found in their experiments that plots those treated with imidacloprid provide maximum yield. However, Naresh and Thakur (1972), Saxena et al. (1984), Chhabra and Kooner (1986) and Vadodaria and Vyas (1987) obtained higher grain yield in oxydemeton methyl treatment. Sethuraman et al. (2001) obtained higher seed yield of greengram with the application of NSKE spray. It may be inferred from the present investigation that yield advantages from the mungbean crop under the existing conditions could be achieved by using the imidacloprid against the sucking insect pest complexes of mungbean.
Data on incremental cost benefit ratio presented in Table 3 revealed that cost effectiveness of treatment T2, seed treatment with dimethoate 5 ml/kg with highest ICBR of 1: 13.41. It was followed by treatment T5, seed treatment with dimethoate + triazophos 0.04% spray (1: 11.93) and treatment T6, seed treatment with dimethoate + triazophos 0.02% spray (1:11.57). Lowest ICBR was estimated in treatment T7, seed treatment with imidacloprid + NSKE 5% spray (1: 6.82). Other promising treatments can be arranged in descending order of cost effectiveness as treatment T4, seed treatment with imidacloprid + triazophos 0.02% spray (1: 7.42), treatment T1, seed treatment with imidacloprid 3 ml/kg (1: 8.62), treatment T8, seed treatment with dimethoate + NSKE 5% spray (1: 9.63) and treatment T3, seed treatment with imidacloprid + spray of triazophos 0.02% (1: 9.94). Similar findings on maximum cost benefit ratio (1: 29.5) was reported by Panghal et al. (2008) in greengram when seed treatment was done by dimethoate 5 ml/kg seed. Contrasting results from the present study are reported by Gupta and Pathak (2009) who reported higher ICBR of 1: 16.9 in NSKE 3% and 1: 11.2 in NSKE 3% followed by spray of dimethoate 0.03%.
Avoidable yield loss (%)
Data on per cent avoidable yield loss depicted in table 1 and revealed that in treatment T5, seed treatment with dimethoate followed by spray of triazophos 0.04%, per cent avoidable yield loss was maximum (68.01%). It was followed by treatment T3, seed treatment with imidacloprid followed by spray of triazophos 0.04% (61.55%) and treatment T8, seed treatment with dimethoate followed by spray of NSKE 5% (57.34%).
Data from the Table 2 shows that application of imidacloprid as seed treatment and spray of triazophos 0.04% registered maximum percent avoidable yield loss (28.09%) and it was followed by 25.12% in seed treatment with dimethoate followed by spray of triazophos 0.04%. While the application of imidacloprid as seed treatment and spray of triazophos 0.02% was unable to reduce the percent avoidable yield loss (9.91%). So, from the findings on per cent avoidable yield loss, it can be concluded that application of triazophos 0.04% could be the first choice against whitefly and reduce the per cent avoidable yield loss in mungbean crop.
The authors have not declared any conflict of interest.