Full Length Research Paper
ABSTRACT
Despite the significance of cassava as food, feed and industrial root crop, little is known regarding the gene action determining root dry matter content (RDMC), fresh root yield, and tolerance to cassava mosaic disease (CMD), cassava green mite (CGM), and cassava mealy bug (CMB). Thus, a study was conducted to determine the general and specific combining abilities for disease, pest, RDMC, root yield and related traits by crossing 10 parents in a 6 × 4 line by tester design. The F1 progenies and their parents were assessed in-field in a randomized complete block design (RCBD) with three replicates. Findings implied sufficient genetic variability for all traits studied. Family TMEB419×IBA030305 had the highest RDMC of 35.47%, whilst family TMEB7×IBA0000203 had the least RDMC (23.87%). Genotypes IBA020588, IBA916132 and TMEB419 were the best parents for improvement of harvest index (HI) and RDMC due to its high positive and significant GCA effects. Genotype IBA000203 contributed the highest to increased plant height, whereas TMEB1, TMEB47 and ZAR010116 had significant negative GCA effects. ZAR010116 was the best tester for HI. Families TMEB778×ZAR010116 (34.23) and IBA020588×ZAR010116 (32.78) were the best performing families for mean RDMC, with parent ZAR010116, exhibiting the highest GCA effect for RDMC. Families TMEB419×ZAR000156, IBA916132×ZAR000156 and IBA020588×IBA000156 had low mean CMD scores of 1.1, 1.2 and 1.2, respectively. The preponderance of non-additive gene actions indicated that selection of superior plants should be postponed to later generation.
Key words: Cassava, genetic improvement, agronomic traits, combining ability, heritability, progeny performance.
INTRODUCTION
Cassava (Manihot esculenta Crantz) is an important staple food crop consumed by over 800 million people worldwide (Esuma et al., 2019). Cassava has varying food, feed and industrial applications that support many livelihoods around the world. In Sierra Leone, cassava is the most consumed root crop. Cassava fresh storage roots are enrich in starch and possess small amounts of calcium (16 mg/100 g), phosphorus (27 mg/100 g), vitamin C (20.6 mg/100 g), minute quantities of protein and other nutrients (USDA, 2016). Moreover, the leaves of cassava are consumed in the country as vegetables since they contain protein such as lysine, but lack the amino acid methionine and possibly tryptophan (FAO, 2010). Other cassava products utilized in the country include cassava pellets for animal feed, cassava starch for sweeteners, thickeners and textile paper industry (Chipeta et al., 2013).
Despite its enormous significance, increased cassava productivity is fraught with a number of biotic and abiotic factors (Kintché et al., 2017). Biotic constraints such as cassava green mite can cause about 15 and 73% yield losses in resistant and susceptible genotypes of cassava, respectively (Bellotti, 2002). Cassava mealy bug damage caused about 88% yield loss in susceptible genotype of cassava (Bellotti et al., 1987). Host plant resistance is strongly advocated for the control of pests and diseases than the continual utilization of pesticides due to its adverse environmental effects on the ecosystem and unsustainability for low-income small-scale farmers (Bellotti, 2002). Environmental variability is also known to contribute to low yields of crops.
In traditional farming systems, cassava is cultivated by stem cuttings and in multiple cropping with other crops, which makes pest control difficult with consequent low yields and wide gap between potential and realized yields. In cassava breeding programmes, botanical seeds are generated through sexual recombination in the first stage (Ceballos et al., 2016). This is done to break the conventional clonal propagation of highly heterozygous cassava genotypes through crossing leading to botanical seed production and increased genetic variation (Grüneberg et al., 2009). Each plant grown from botanical seed is a potential new variety studied during the selection cycle (Ceballos et al., 2016). The efficiency of selection depends on the breeders’ ability to identify useful variability created in existing and new improved populations. A robust breeding strategy that utilizes locally adapted cultivar(s) with wide adoptability in crosses for their genetic improvement for desired traits of interests may help the breeder in identifying the fewer useful crosses needed (Witcombe and Virk, 2001). Such few but “smart or clever” crosses, involving parents with desired complimentary traits is an established concept utilized in the generation of useful genetic diversity in cassava (Manu-Aduening et al., 2013).
The line × tester mating design is one of such breeding strategies that simultaneously produces both full-sibs and half-sibs. The design provides specific combining ability (SCA) of each cross, and general combining ability (GCA) of the lines and testers, with both the lines and testers exhibiting different sets of genotypes (Farhan et al., 2012). The concept of combining ability was first introduced by Sprague and Tatum (1942). Combining ability is important in plant breeding because it provides information on the nature and magnitude of gene action and for the selection of parents (Solongi et al., 2019). It comprises two types including the GCA and SCA. The GCA is the mean performance of a parent in a series of crosses, whereas SCA denotes cross combinations that perform relatively better or worse than expected mean performance of lines involved. A significant line × tester interaction provides evidence that the differential ranking of experimental lines depends on the particular tester used (Packer, 2007). This necessitates use of appropriate tester for evaluation of new germplasm lines (Ali et al., 2011). The testers used in a breeding programme may either be genetically narrow or broad-based, related or unrelated to the lines being evaluated or may have high or low frequency of favourable alleles and high or low yielding (Ali et al., 2011). The combining ability between the line and the tester determines the performance of the progenies (Fasahat et al., 2016). Thus, lines with good performances are advanced to the next breeding stage, whereas those with poor combining abilities are discarded.
There is increasing demand on cassava productivity for food, feed and industrial uses that necessitates the continued improvement of cassava for high yield, dry matter content, and food and market quality traits. Improvement of cassava for desired traits require understanding of mode of gene action controlling these traits. In Sierra Leone, no genetic studies have been done on the mode of gene action controlling the expression of pests, diseases, dry matter content, yield and related attributes. Such information would facilitate the efficient selection of superior genotypes and designing efficient cassava improvement programme that incorporate economic traits that enhance sustainable cassava production and productivity of cassava producers in Sierra Leone. The objectives of this study were to (i) determine the general and specific combining abilities and broad sense heritability estimates for cassava mealy bug, cassava green mite, cassava mosaic disease, root dry matter content, fresh root yield and related attributes for selection of elite genotypes; and (ii) determine the phenotypic associations among agronomic traits in cassava.
MATERIALS AND METHODS
Site description
The crossing trial was conducted in early May, 2015 at the Ubiaja (8°29'N, 76°57'E, 64 m altitude) crop site of the International Institute of Tropical Agriculture (IITA), Nigeria. The mean annual rainfall at Ubiaja was 1741.2 mm, relative humidity of 86.0 and mean minimum and maximum temperature of 22.5 and 29.1°C, respectively. The seedling nursery trial was done at the Njala Agricultural Research Centre (NARC) experimental site, Njala (8°06′N latitude and 12°06′W longitude and elevation of 50 m above sea level) southern Sierra Leone during the 2016/2017 cropping season. The mean annual rainfall at Njala was 2525 mm; mean monthly maximum air temperature range from 23 to 29°C; and relative humidity ranged from 80 to 100%.
Plant materials and experimental design
Ten genetically diverse parents comprising six lines (males) and four testers (females) from the genetic gain trial cassava breeding programme at International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria were used (Table 1). The parents were crossed in a 6×4 line × tester mating design to produce 24 F1 families. Controlled pollinations were performed following the standard procedures described by Kawano (1980). The F1 seeds and parents were planted in-field in early May in a randomized complete block design with three replications. Each plot consisted of a single row with seeds planted at a spacing of 0.3 m × 1 m, between and within rows, respectively. Each row comprised 20 seeds. The recommended timely weeding was done.
Data collection
Data collected during the trial included diseases and pests: cassava mosaic disease (CMD), cassava bacterial blight (CBB) and cassava green mite (CGM) at six months after planting (MAP) using a scale of 1-5, where: 1 = no symptoms and 5 = very severe mosaic symptoms (Banito et al., 2007). Data collected at harvest (11 MAP) included: plant height (PHT), harvest index (HI), number of storage root (NSR) and fresh storage root weight (FSRW) (kg plant-1) and root dry matter content (RDMC). For PHT, 10 plants were measured per plot using a meter rule as the distance from the ground to the shoot tip. Storage roots plant-1 were counted and weighed to obtain number of storage root (NSR) and fresh storage root weight (FSRW) (kg plant-1), respectively.
The RDMC was determined by selecting two representative roots from the bulk roots per plant, peeling, washing, slicing and oven-drying 100 g per sample at 65 to 70°C till a constant weight is obtained at about 72 h (Fukuda et al., 2010). The fresh storage roots were washed and shredded into pieces. The DMC was calculated as:
where RDMC = root dry matter content expressed as a percentage; DRM = dry root mass (kg), and FRM = fresh root mass (kg).
Data analysis
The data collected for all traits of the 24 families were analyzed using the General Linear Model procedure (PROC GLM) of SAS version 9.4 (SAS, 2013). Mean squares were calculated from type III sum of squares. Genotypes were partitioned into replication, lines, tester, line × tester and crosses. Further genetic analyses were carried out for traits among the progeny according to line × tester analysis methods as suggested by Kempthorne (1957) to partition the mean square due to crosses, lines (GCAf), tester (GCAm) and line × tester interactions (SCAfm). The contributions of the traits to the total variability of the 24 families were analyzed according to Jollife (2002), using principal component analysis (PCA) in SAS version 9.4 (SAS, 2013). Pearson’s phenotypic correlations between the 24 family means for each trait were also performed using SAS version 9.4 (SAS, 2013). The linear model for line × tester design used was:
where  = observed value of the cross i×j in the kth replication;
= observed value of the cross i×j in the kth replication; 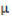 = population mean effect;
= population mean effect; 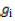 = GCA effect of ith tester;
= GCA effect of ith tester; 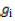 = GCA effect of jth line;
= GCA effect of jth line; 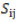 = SCA effect of the cross i×j;
= SCA effect of the cross i×j;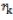 = effect of the kth block;
= effect of the kth block; 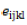 = experimental error due to (ijk)th individual.
= experimental error due to (ijk)th individual.
The variances for general and specific combining ability were tested against their respective error variances, derived from the analysis of variance of the different traits as follows:
With the assumption of no epistasis, variances due to GCA 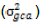 and variance due to SCA
and variance due to SCA 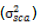 are estimated as follows:
are estimated as follows:
Additive and dominance genetic variances  were calculated by taking inbreeding coefficient (ð¹) equal to one; that is, ð¹ = 1 because both lines and testers were inbred.
were calculated by taking inbreeding coefficient (ð¹) equal to one; that is, ð¹ = 1 because both lines and testers were inbred.
Significance test for general combining ability and specific combining ability effects were performed using ð‘¡-test.
Broad sense heritability on mean entry basis was estimated as the ratio of genotypic variance to the phenotypic variance and expressed in percentage as described by Robinson et al. (1949).
where 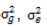 and
and 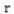 represent the genetic variance, environmental variance, and number of replications, respectively. The broad sense heritability was classified based on the scale described by Robinson et al. (1949): low (0 - 30%); moderate (30 - 60%); and high >60%.
represent the genetic variance, environmental variance, and number of replications, respectively. The broad sense heritability was classified based on the scale described by Robinson et al. (1949): low (0 - 30%); moderate (30 - 60%); and high >60%.
RESULTS
Analysis of variance for the line × tester design crosses
Analysis of variance showed that mean squares due to crosses were significant for traits such as PHT, NSR, CBB, FSRW and HI and highly significant for CGM (Table 1). Mean squares values for line were highly significant for CGM and significant for PHT, NSR, CBB, FSRW, HI and RDMC. The mean squares values for testers were significant for PHT, CMD, CBB and HI. The line × tester interactions were highly significant for CGM and significant for CBB, FSRW and HI. The differences among the replications were not significant for any of the traits.
The broad sense heritability estimates were high for all traits ranging from 75.0 to 90.8% (Table 2).
Mean performances of progenies of 24 families
Progenies of family TMEB778×IBA000203 (208.6 cm) had the highest mean PHT, whereas family TMEB1×ZAR010116 (141.5 cm) had the lowest (Table 3). The mean NSR ranged from 1.73 (TMEB1×IBA000203) to 4.80 (IBA916132×IBA000203). The heaviest mean performance for FSRW was observed in family TMEB7×ZAR010116 (2.23 kg), whilst family TMEB778×ZAR000156 (0.90 kg) had the lightest weight. The mean performance for HI ranged from 0.29 (TMEB1×IBA0000203) to 0.44 (TMEB778×ZAR000156). Family TMEB7×IBA0000203 exhibited the least mean performance for RDMC (23.87%), whereas TMEB419×IBA030305 had the highest (35.47%).
The least mean score of 1.10 for CMD was recorded by TMEB419×ZAR010156 (family of parents tolerant to CMD) and the highest of 1.77 by IBA020588×IBA030305 (family of a cross between parents susceptible to CMD). The highest mean score for CGM of 2.53 was recorded by TMEB778×IBA000156 and the least score of 1.60 was recorded by TMEB7×ZAR010116. The highest mean score of 2.97 for CBB was recorded by IBA916132×IBA000203, whereas TMEB419×IBA030305 had the least score of 1.67 (Table 3).
General combining ability
The GCA for lines and testers are shown in Table 4. The line GCA effects for PHT was negative and significant for TMEB1 (-16.98 cm) and TMEB7 (-11.01cm) and positive and significant for TMEB778 (10.29 cm) and TMEB419 (9.88 cm), at (p<0.05). With respect to NSR, line IBA916132 showed positive and significant GCA effects (0.51), whereas line TMEB1 showed significant and negative GCA effect (-0.77). Significant and positive GCA effects were observed in IBA020588 (0.14) and TMEB778 (0.24); and TMEB778 significant and negative GCA effects in TMEB7 (-0.29) for CMD severity at 6 MAP. TMEB419 and TMEB7 showed significant and negative GCA effects of -0.26 for CBB severity at 6 MAP. Estimates of GCA effects for FSRW showed that TMEB7 exhibited positive and significant GCA effects of 0.46 kg while TMEB778 exhibited negative and significant GCA effects of -0.34 kg. The GCA effects for HI showed that IBA020588, IBA916132, TMEB419 and TMEB778 exhibited positive and significant GCA effects of 0.02, 0.03, 0.04 and 0.02, respectively. IBA020588, IBA916132 and TMEB419 exhibited significant (p<0.05) and positive GCA effects of 1.06, 1.17 and 2.32, respectively, for RDMC.
Tester IBA000203 had a significant and positive GCA effect for PHT (12.23) and significant and negative GCA effects (-1.36) for RDMC (Table 3). IBA030305 exhibited a significant and negative GCA effect for CBB severity (-0.13) and a significant and positive GCA effects (0.17) for FSRW. ZAR000156 had significant and negative GCA effects of -0.16 and -0.12 for CMD severity and CBB severity, respectively. Genotype ZAR010116 had significant and negative GCA effects of -16.23 for PHT and significant and positive GCA effects for CBB severity (0.23), HI and RDMC.
Specific combining ability
Families IBA020588×ZAR000156 and TMEB7×ZAR000156 recorded a negative and significant SCA effects for PHT (Table 5). Positive and significant SCA effects were observed for NSR in families IBA916132×IBA000203, TMEB419×IBA000203, TMEB7×IBA030305 and TMEB1×ZAR000156; whereas families TMEB1×IBA000203, TMEB7×IBA000203, TMEB778×IBA030305 and IBA916132×ZAR010116 had negative and significant values. However, the highest and positive SCA effects for NSR were observed in families TMEB7×IBA030305, TMEB1×ZAR000156 and IBA916132×ZAR010116. Family TMEB778×IBA000203 showed significant and negative SCA effects for CMD, while TMEB778×ZAR000156 showed a significant and positive SCA effect. Families with negative and significant SCA for CGM were TMEB419×IBA000203, TMEB778×IBA000203, TMEB1×IBA030305 and IBA020588×ZAR000156.
With respect to CBB, both positive and negative SCA effects were recorded with significant difference at (p<0.01) and (p<0.05), with the highest positive SCA effect recorded for family IBA916132×IBA000203. Three families (TMEB778×IBA000203, IBA020588×ZAR010116 and IBA916132×ZAR010116) showed significant and negative SCA effect for CBB. Families IBA020588×IBA000203, TMEB778×IBA030305, TMEB778×IBA000203, IBA020588×ZAR010116 and TMEB419×ZAR010116 recorded negative SCA effects for FSRW; while TMEB778×IBA000203 and IBA020588×ZAR010116 were significant and positive. Significant and positive SCA effect for HI was observed in IBA020588×IBA000203, IBA916132×IBA000203, TMEB7×IBA030305 and TMEB778×ZAR000156. The RDMC showed significant (p<0.05) positive SCA for families IBA916132×IBA000203, TMEB419×IBA030305, TMEB1×ZAR000156 and TMEB778×ZAR010116 (Table 4).
Phenotypic correlation coefficients of agronomic traits
The phenotypic correlation coefficients vary for the various agronomic trait associations studied (Table 6). Cassava bacterial blight (CBB) was positively correlated with CGM; whereas FSRW was significant and negatively correlated with CGM and CBB. Harvest index (HI) was significantly and positively correlated with NSR, CMD, CGM, and CBB and negatively correlated with FSRW.
DISCUSSION
Combining ability effects of agronomic traits
Traits of testers, lines and families that exhibited significant GCA and SCA mean square values indicate the preponderance of significant variations in the breeding populations, possibly attributable to additive and non-additive gene effects, respectively. Kamau et al. (2010) and Parkes (2011) also found significant effects for root number per plant and fresh root weight per plant. However, findings of the current study disagree with the non-significant GCA values reported for average root number and fresh root weight by DaSilva (2008). The variance is partly attributable to the different populations developed and environments in which they were assessed. In this study, the GCAs of CMD for all lines and GCAs of number of storage roots and CGM for all testers were non-significant.
Genotypes with significant negative GCA values for CMD, CGM, CMB and CBB indicate that they possibly possess desirable alleles for resistance to CMD, CGM, CMB and CBB that are needed in the development of new varieties resistant to the studied pests and diseases. Thus, lines and testers possessing these attributes are useful parental genotypes for cassava population improvement aimed at generating resistant genotypes to the biotic constraints (Owolade et al., 2008).
Genotypes with significant positive GCA values for plant height, root number per plant, fresh root weight per plant, harvest index and dry matter content were considered superior genotypes that contributed most to variability in the traits, while those with significant negative values were undesirable since they performed below average. Since none of the lines and testers used in this study possessed the overall best general combiner attributes for all the traits that qualify it for the improvement of all the traits in a breeding program, indicate the relevance of recombination to incorporate desired traits from parents with complementary desired alleles of traits of interests.
The distribution of family (pedigree of F1 progenies) for the studied traits relative to GCA values of parental combinations showed that most of the SCA effects were obtained from different GCA values rather than from best general combiners indicating that the inheritance of these traits involved both allelic and non-allelic interactions. These findings concurred with those reported by Saleem (2008). For instance, family TMEB419 × IBA030305 (high × low), TMEB7 × IBA030305 (low × low), TMEB778 × ZAR000156 (low × intermediate) combinations for dry matter content, IBA020588×ZAR000156 (high × intermediate), TMEB778 × IBA030305 was obtained from (high × low), TMEB7×IBA030305 (low × low) combinations for CGM. Based on the performances of hybrids depicted by the SCA values, best progenies might not always be obtained from crosses among parents with highest desirable GCA effects. These findings concurred with the suggestion by other researchers that best progenies are not always obtained from parental crosses with the highest desired GCA effects (DaSilva, 2008; Owolade et al., 2008; Mtunda, 2009; Kamau et al., 2010).
Gene action and significance of general and specific combining abilities
Plant height had lower general combining ability relative to specific combining ability indicating that the SCA was more important in predicting progeny performance for expression of this trait. However, the remaining traits exhibited higher GCA relative to SCA except for CGM, indicating the relevance of GCA in predicting progeny performance for the expression of the traits. Findings partly agree with DaSilva (2008) and Parkes (2011) who reported that SCA was more important for prediction of progeny performances of cassava. Since GCA and SCA effects were relevant for traits studied, findings agree with the suggestion that GCA and SCA gene action effects should be part of breeding schemes targeted at selection of superior genotypes (Arunga et al., 2010). Since cassava is a highly heterozygous crop cultivated using vegetative propagules such as stem cuttings, identification and selection of superior clones with desired traits can be perpetuated intact through the various breeding stages.
Phenotypic correlation and heritability estimates of agronomic traits
The positive correlation between fresh storage root weight and root number per plant implies that indirect selection for storage root weight is achievable to certain level by selecting for root number and that decrease in root storage number contributes to decreasing yields in cassava. This result is in concurrence with those noted by DaSilva (2008), Akinwale et al. (2009, 2010) and Kamau et al. (2010). Similarly, positive relationships between other economic traits were: dry matter content and root number per plant, dry matter content and fresh storage root weight, dry matter content and harvest index, and between root number per plant and harvest index.
The negative correlations between CMD and fresh root weight, CGM and fresh root weight, and between CBB and fresh root weight, indicate that severe attacks of these diseases contribute to low storage root yields in cassava. Findings agree with Bellotti (2002), who noted the impact of CGM on cassava root yield. Yield reduction by any biotic factor can be decreased through incorporation of host plant resistance in cassava breeding programme. Moreover, a significant negative association between harvest index and fresh root weight also indicates that higher fresh root yields are achieved by decreasing harvest index. The results are consistent with Akinwale et al. (2009) who opined that traits that are significantly and positively related with fresh storage root yield are important in the formulation of an efficient cassava breeding programme aimed at improving fresh root yield, while negatively correlated ones may reduce the rate of improvement for some traits under selection in a breeding programme.
The high heritability in the measured traits of the studied plants indicates the preponderance of larger genetic effects contributing to the total phenotypic variance and that the alleles of these traits could be passed intact to subsequent generations of the cassava root crop. Our findings are consistent with the high broad sense heritability values reported for these traits in cassava (DaSilva, 2008; Akinwale et al., 2010; Parkes, 2011). Findings corroborate with the suggestion by DaSilva (2008) that broad-sense heritability predominates in cassava hybrids. Thus, the high broad sense heritability in the studied progenies implies that a large proportion of the heritable variation could be exploited by plant breeders (Akinwale et al., 2010).
CONCLUSION
Genotypes with good GCA and cross combinations with desirable SCA for the traits studied were identified. Parental genotypes TMEB419, IBA020588, BA916132 and ZAR010116 had good general combining ability for dry matter content, whereas TMEB7 and IBA030305 exhibited good general combining ability for storage root weight. Among the testcrosses, TMEB778×ZAR010116 and TMEB419×IBA030305 were good specific combiners for dry matter content and could be used for heterosis breeding programmes in cassava.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors are grateful to the cassava breeding team at the International Institute of Tropical Agricultural Nigeria (IITA) for their technical support during the course of this study. This study was funded by the West Africa Agricultural Productivity Program Sierra Leone (WAAPP 1C SL) (IDA Grant H654-SL), Japan (PHRD TF099510-SL) and the International Development Research Centre (IDRC) under the IDRC/CORAF-WECARD/IITA sub-grant agreement for developing capacity for Agricultural Research in Sub-Saharan Africa (PJ-2126).
REFERENCES
|
Akinwale MG, Akinyele BO, Dixon AGO, Odiyi AC (2009). Genetic variability among cassava genotypes in three agro-ecology zones of Nigeria. African Crop Science Conference Proceedings 9:541-546. |
|
|
Akinwale MG, Aladesanwa RD, Akinyele BO, Dixon AGO, Odiyi AC (2010). Inheritance of ß-carotene in cassava (Manihot esculenta Crantz). International Journal of Genetics and Molecular Biology 2(10):198-201. |
|
|
Ali F, Muneer M, Rahman H, Noor M, Durrishahwar SS, Yan J (2011). Heritability estimates for yield and related traits based on testcross progeny performance of resistant maize inbred lines. Journal of Food, Agriculture and Environment Research Letter 9:438-443. |
|
|
Arunga EM, Van Rheenen HA, Owuoche JO (2010). Diallel analysis of Snap bean (Phaseolus vulgaris L.) varieties for important traits. African Journal of Agricultural Research 5(15):1951-1957. |
|
|
Banito AJ, Verdier V, Kpemoua KE, Wydra K (2007). Assessment of major cassava diseases in Togo in relation to agronomic and environmental characteristics in a systems approach. African Journal of Agricultural Research 2:418-428. |
|
|
Bellotti AC (2002). Arthropod pests. In: R.J. Hillocks JM, Thresh AC, Bellotti (Eds.). Cassava: Biology, production and utilization. London, UK: CABI Publishing, pp. 209-235. |
|
|
Bellotti AC, Hershey CH, Vargas O (1987). Recent advances in resistance to insect and mite pests of cassava. In: Hershey, C.H. (ed.) cassava breeding: A multidisciplinary review, proceedings of a workshop held in Philippines, 4-7 March, 1985. CIAT, Cali, Colombia pp. 117-146. |
|
|
Ceballos H, Pérez JC, Barandica OJ, Lenis JI, Morante N, Calle F, Pino L, Hershey CH (2016). Cassava Breeding I: The Value of Breeding Value. Frontiers in Plant Science 7:1227. |
|
|
Chipeta MM, Bokosi JM, Saka VW, Benesi IRM (2013). Combining ability and mode of gene action in cassava for resistance to cassava green mite and cassava mealy bug in Malawi. Global Science Research Journals 1(1):071-078. |
|
|
DaSilva AMZ (2008). Breeding potential of cassava (Manihot esculenta Crantz) in Mozambique (PhD Thesis). University of the Free State, South Africa. |
|
|
Esuma W, Nanyonjo AR, Milro R, Angudubo S, Kawuki RS (2019). Men and women's perception of yellow-root cassava among rural farmers in eastern Uganda. Agriculture and Food Security 8(10):1-9. |
|
|
Food and Agricultural Organization (FAO) (2010). "Preparation of cassava leaf products and their use as animal feeds". FAO Animal Production and Health Paper 95:111-125. Retrieved 13 August 2010. |
|
|
Farhan A, Irfan Ahmed S, Hidayat UR, Mohammad N, Durri S, Muhammad YK, Ihteram U, Jianbing Y (2012). Heterosis for yield and agronomic attributes in diverse maize germplasm. Australian Journal of Crop Science 6:455-462. |
|
|
Fasahat P, Rajabi A, Rad JM, Derere J (2016). Principles and utilization of combining ability in plant breeding. Biometrics and Biostatics International Journal 4(1):1-22. |
|
|
Fukuda WMG, Guevara CL, Kawuk R, Ferguson ME (2010). Selected morphological and agronomic descriptors for the characterization of cassava. International Institute of Tropical Agriculture (IITA): Ibadan (Nigeria) 19 p. |
|
|
Grüneberg W, Mwanga R, Andrade M, Espinoza J (2009). Selection methods Part 5: Breeding clonally propagated crops. In: Ceccarelli S, Guimaraes EP, Weltizien E (eds.). Plant breeding and farmer participation, Chapter 13, Food and Agriculture Organization, Rome, Italy pp. 275-366. |
|
|
Jollife IT (2002). Principal component analysis. Second edition, Springer-Verlag New York, Inc. USA. |
|
|
Kamau J, Melis R, Laing M, Derera J, Shanahan P, Ngugi E (2010). Combining the yield ability and secondary traits of selected cassava genotypes in the semi-arid areas of Eastern Kenya. Journal of Plant Breeding and Crop Science 2(7):181-191. |
|
|
Kawano K (1980). Cassava. In: Fehr WR, Hadley HH (eds.) Hybridization of crop plants. ASA, CSSA, Madison, Wisconsin, USA pp. 225-233. |
|
|
Kempthorne O (1957). An introduction to genetic statistics. John Wiley and Sons, Inc., New York. |
|
|
Kintché K, Hauser S, Mahungu NM, Ndonda A, Lukombo S, Nhamo N, Uzokwe VNE, Yomeni M, Ngamitshara J, Ekoko B, Mbala M, Akem C, Pypers P, Matungulu KP, Kehbila A, Vanlauwe B (2017). Cassava yield loss in farmer fields was mainly caused by low soil fertility and suboptimal management practices in two provinces of the Democratic Republic of Congo. European Journal of Agronomy 89:107-123. |
|
|
Manu-Aduening JA, Peprah BB, Agyeman A (2013). Genetic variability of cassava progenies developed through introgression of cassava mosaic disease resistance into Ghanaian landraces. Journal of Crop Science and Biotechnology 16(1):23-28. |
|
|
Mtunda KJ (2009). Breeding, evaluation and selection of Cassava for high starch content and yield in Tanzania (PhD Thesis). University of KwaZulu-Natal, Pietermaritzburg, South Africa. |
|
|
Owolade OF, Dixon AGO, Alabi BS, Akande SR, Olakojo SA (2008). A combining ability analysis of cassava (Manihot esculenta Crantz) genotypes to anthracnose disease. Electronic Journal of Environmental, Agricultural, Food Chemistry 7(6):2959-2968. |
|
|
Packer DJ (2007). Comparing the performance of F1 testers versus their inbred line parents in evaluating experimental sorghum R and B lines in testcrosses. Master of Science Plant Breeding: Brigham Young University. |
|
|
Parkes EY (2011). Assessment of genetic diversity, combining ability, stability and farmer preference of cassava germplasm in Ghana (PhD Thesis), University of the Free State, South Africa. |
|
|
Robinson HF, Comstock RE, Harvey PH (1949). Estimates of heritability and the degree of dominance in corn. Agronomy Journal 41(8):353-359. |
|
|
Saleem MY (2008). Genetic analysis of Basmati Rice (Oryza sativa L.) (PhD Thesis). Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan P 160. |
|
|
SAS Statistical Analysis System Institute Incorporated (SAS) (2013). SAS for Windows 9.4. Cary, NC: SAS Institute Inc. |
|
|
Solongi N, Jatoi WA, Baloch MJ, Siyal M, Solangi AH, Memon S (2019). Heterosis and combining ability estimates for assessing potential parents to develop f1 hybrids in upland cotton. The Journal of Animal and Plant Sciences 29(5):1-12. |
|
|
Sprague GF, Tatum LA (1942). General versus specific combing ability in single crosses of corn. Journal of American Society of Agronomy 34:923-932. |
|
|
USDA United States Department of Agriculture (2016). "Basic Report: 11134, Cassava, raw". National Nutrient Database for Standard Reference Release 28. Agricultural Research Service, US Department of Agriculture. May 2016. Retrieved 7 December 2016. |
|
|
Witcombe JR, Virk DS (2001). Number of crosses and population size for participatory and classical plant breeding. Euphytica 122:451-462. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0