ABSTRACT
Calcareous soil, comprising more than 15% CaCO3, which imposes various physical and chemical challenges to plants, is widespread in arid and semi-arid regions. It was against the stated backdrop that an experiment was carried out to compare growth responses of moringa (Moringa oleifera Lam.) seedlings in calcareous, clayey and sandy soils relative to those in loamy soil during autumn (January-March) 2015 and validated in 2016. Treatments comprised loamy, calcareous, clayey and sandy soils, arranged in randomised complete block design, with 15 replications. Each soil type was steam-pasteurised, with hardened-off six-week-old seedlings transplanted in 10 L plastic bags containing 9.8 L soil for each. At 90 days after transplanting, treatments had significantly (p ≤ 0.05) affected growth variables of M. oleifera seedlings. Relative to loam soil, calcareous soil reduced dry shoot mass (33%), chlorophyll content (36%) and dry root mass (47%), but increased root length (28%). In contrast, clay increased dry shoot mass (66%), leaf number (25%) and root length (26%), whereas sandy soil increased dry shoot mass (42%) and reduced dry root mass (51%). In conclusion, relative to loam soil, marginal soils such as calcareous, clayey and sandy soils had effects on growth of M. oleifera seedlings that included physical and chemical attributes of the soil types.
Key words: Calcareous soil, marginal soil, Moringa species, soil pH, soil texture, soil type.
Moringa (Moringa oleifera Lam.) tree, dubbed the “miracle tree” (Fuglie, 2001), due to its social, economic, environmental, nutritional and medicinal versatilities, is being adopted at the fastest rate as a cultigen (Mridha, 2015). M. oleifera projects in marginal communities are being established on marginal soils such as heavy clay, infertile sandy, sodic and calcareous soils (Foidl et al., 2001). Although soil depth is the most limiting descriptor of marginal soils, physical, chemical and biological features impose serious limitations on crop production (FAO, 1973). Loam soil is an ideal soil type for M. oleifera husbandry (Ramachanran et al., 1980; Bezerra et al., 2004). However, others claimed that the species was well-adapted to a wide range of soil types (Mridha, 2015). The genus Moringa, with thirteen species (Leone et al., 2015), had Moringa drouhardii as being the only species that originated in Madagascar Province on calcareous soils (Leone et al., 2015), with M. oleifera being grown widely in marginal communities.
Clayey soils have good attributes in terms of retention of essential nutrients and moisture, but could affect plant growth through its high resistance to movement and waterlogging (Brady and Weil, 2009). Empirical evidence suggested that M. olefeira could excel in clay and sand (Pahla et al., 2013) and sodic (Hegazi, 2015) soils, without limited information for calcareous soil. The latter is defined as soil with CaCO3 greater than 15%, which could exist in various forms (FAO, 1973). Globally, calcareous soil is widely distributed in arid and semi-arid regions (FAO, 1973) and could impose challenges in crop husbandry due to its high CaCO3, which limits and promotes the availability of certain nutrient elements (FAO, 1973; Kabata-Pendias, 2010).
Soil type studies on M. oleifera had been limited to sodic soil (Hegazi, 2015), sand and clay soils (Pahla et al., 2013; Hegazi, 2015), with evidence that the crop is tolerant to these marginal conditions. Although calcareous soil is widely distributed in marginal communities where M. oleifera is widely adopted for food security, job creation and wealth generation, there is hardly any evidence that the influence of this soil on growth of M. oleifera had previously been tested. The objective of this study was to determine the relative effects of calcareous, clay and sandy soils on growth of M. oleifera in comparison with effects of loam soil.
Study location and preparation of materials
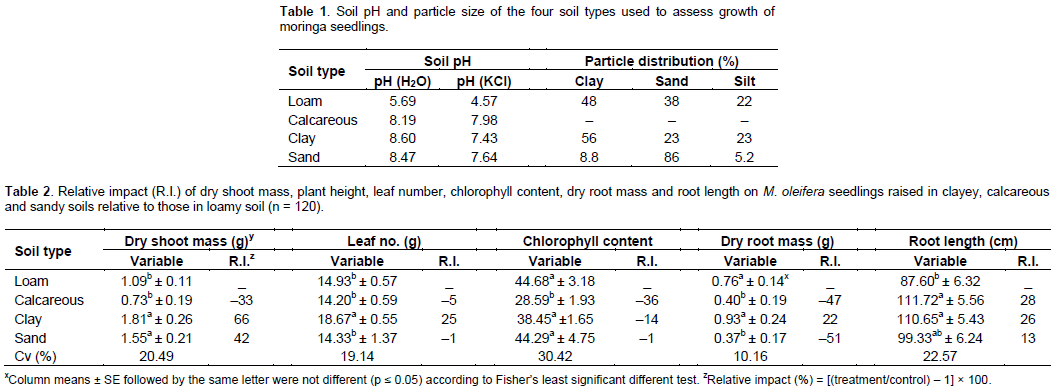
The experiment was conducted in the greenhouse at the Green Biotechnologies Research Centre of Excellence, University of Limpopo, South Africa (23°53'10"S, 29°44'15"E) during autumn (January-March) 2015 and validated in 2016. Ambient maximum/minimum temperature averaged 28/22°C, with day temperature controlled using thermostatically-activated fans. Loam, clay and calcareous soils were collected at the Centre, Madisha-Ditoro town (29°42'20"S, 30°56'32"E) and Moletlane (24°20'59"S, 29°19'14"E) town. The soil pH [1:2 soil and water (v/v) ratio] and particle size were quantified using Thomas (1996) and Bouyoucos (1962) methods, respectively. Due to calcareous soil-related challenges in quantifying soil particle size (FAO, 1973), the variable was not quantified.
Seedlings were raised in 160-hole seedling trays using Hygromix-T (Hygrotec, Pretoria) and hardened-off for a week prior to replanting. At three-leaf stage, seedlings were hardened-off outside the greenhouse through intermittent withholding of irrigation water. Four soil types were each steam-pasteurised at 300°C for 2 h, 10 L plastic bags were potted with 9.8 L soil of each soil type and placed on the greenhouse benches at 0.2 m inter-row and 0.2 m intra-row spacing. Uniform six-week-old seedlings of M. oleifera were transplanted, with the soil type arranged in a randomised complete block design, with 15 replications.
Cultural practices
At transplanting, the topsoil of each pot was amended once with 5 g 2:1:2 (43) Multifeed fertiliser to provide a total of 0.88 mg N, 0.88 mg K, 0.40 mg P, 2.25 mg Mg, 1.88 mg Fe, 0.19 mg Cu, 0.88 mg Zn, 2.5 mg B, 7.50 mg Mn and 0.18 mg Mo per ml water. Transplants were irrigated to field capacity at transplanting and then with 250 ml tap water every other third day until the sixth week. Thereafter, transplants were irrigated with 350 ml tap water until harvest.
Data collection
At 90 days after initiating the treatments, plant height was measured from the soil surface to the tip of the flag leaf. Chlorophyll content was measured using a digital chlorophyll meter (SPAD) on the third mature leaf below the flag leaf, with leaf number per plant being recorded. Shoots were severed at the soil surface and the stem diameters were measured at 5 cm above the severed ends using a digital Vernier caliper (Model: DC-515). Roots were removed from the plastic containers, washed with running water to remove soil particles and root length was measured. Shoots and roots were dried in air-forced ovens at 70°C for 72 h and weighed.
Data analysis
Data were subjected to analysis of variance (ANOVA) through the SAS software. Leaf number data were transformed [log10(x + 1)] prior to ANOVA to homogenise the variances (Gomez and Gomez, 1984), but untransformed data were discussed. Mean separation for significant treatment effects was achieved through Fisher’s least significant difference test at the probability level of 5%. Unless stated otherwise, the discussed treatments were significant at the probability level of 5%.
Seasonal effects on variables were assessed and because there were no seasonal interactions, the data were pooled (n = 120). Soil pH (H2O) in loam was less than 6 units, whereas in other soils, it was above 8 units, with distinct (except for calcareous soil) particle sizes (Table 1). Soil type had highly significant (p ≤ 0.01) effects on dry shoot mass, leaf number, chlorophyll content, dry root mass and root length, but had no significant effects on plant height and stem diameter (data not shown). Dry shoot mass of plants grown on clay and sandy soils were not different, but were significantly higher than for those on loam and calcareous soil (Table 2). Relative to loam, calcareous soil reduced dry shoot mass, chlorophyll content and dry root mass by 33, 36 and 47%, respectively, but increased root length (Table 2). Relative to loam soil, clay soil increased dry shoot mass, leaf number and root length by 66, 25 and 26%, respectively (Table 2). In contrast, sandy soils increased dry shoot mass by 42% (Table 2) and reduced dry root mass by 51% (Table 2).
Effects of calcareous soils
Loam soil, due to its optimal role in soil health (chemical, physical and biological), attributes relative to other soil types (Marschner, 1995) was selected for use as a standard. The reduction of dry shoot mass and dry root mass on M. oleifera seedlings in calcareous soils had limited comparative empirically-based studies. In contrast, the significant reduction in chlorophyll content in plants grown in calcareous soil relative to loam soil, suggested that in the long-term, calcareous-grown seedlings would experience photosynthetically active radiation-related challenges as observed in most calcareous soils (Obreza et al., 2015) and salt-affected soils as previously observed on M. oleifera seedlings (Hegazi, 2015). The observed reduction in growth of M. oleifera seedlings in calcareous soil was in agreement with the high soil pH, which could result in N, P, Fe, Mn and Cu being in unavailable forms, whereas K, S, Ca, Mg and Mo could occur in luxurious to phytotoxic concentrations (Marschner, 1995; Obreza et al., 2015). The reduction in plant growth variables of M. oleifera in calcareous soil could be associated with the imbalances of essential nutrient elements as had been observed on most other plant species in similar soils (Obreza et al., 2015).
Although, it could not be justified to linkup one particular nutrient element to the observed growth responses in this study, it is probable that such ionic imbalances in calcareous soils, along with poor soil aggregates, contributed negatively to plant growth as observed on other crops (FAO, 1973). Magnesium and N for instance, are part of the constituents of chlorophyll molecules and calcareous soils are generally low in the two elements (Obreza et al., 2015).
Increased root length in M. oleifera seedlings on calcareous soil in the current study confirmed root growth in other plant species exposed to excessively high Ca ions. Among numerous physiological activities in plants, Ca is associated with mitosis and therefore, cell growth (Hepler, 2005) and by extrapolation, Ca is associated with root elongation. In calcareous soils, despite challenges that reduced most plant growth variables, the observed increase in root length could be associated with the luxurious availability of Ca for cell division in such soils (McMahon et al., 2005).
Effects of clay soil
In contrast to calcareous soil, relative to loam soil in the current study, clay soil increased dry shoot mass, leaf number and root length in M. oleifera seedlings. The findings confirmed observations where M. oleifera seedlings in clay soil had the highest total plant dry matter (Hegazi, 2015; Pahla et al., 2013). Clay soil is renowned for its excellent cation exchange and water-holding capacities, which under best agricultural practices are known to improve plant growth. In the current study, clay soil improved leaf number, which confirmed observations that dry leaf mass on plants grown in clay soil were significantly higher than of those in sandy soil (Hegazi, 2015). Generally, the decreasing effect on aboveground growth of M. oleifera growing on clay was reversed when the soil was amended with cattle manure (Pahla et al., 2013).
Effects of sandy soil
In the current study, sandy soils increased (42%) dry shoot mass, which confirmed other observations where M. oleifera seedlings in sandy soil had the highest total plant dry matter (Heiga, 2015; Pahla et al., 2013). The significant reduction of dry root mass on M. oleifera seedlings in sandy soil in this study confirmed observations where the variable was the lowest in sandy soils (Pahla et al., 2013; Hegazi, 2015). In M. oleifera production, amendment of marginal soils with extreme particle sizes (clay or sand) was managed using organic matter (Pahla et al., 2013) or inorganic fertilisers (Dania et al., 2014). However, challenges imposed by calcareous soil in relation to high CaCO3 had been rather difficult to manage in crop husbandry (Obreza et al., 2015).
Findings in the current study demonstrated that the marginal soils such as calcareous, clayey and sandy soils had both negative and positive attributes on growth of M. oleifera seedlings. Relative to loam soil, calcareous soil reduced dry shoot mass, chlorophyll content and dry root mass, but increased root length. In contrast, clay increased dry shoot mass, leaf number and root length; whereas sandy soils increased dry shoot mass, but reduced dry root mass. In conclusion, relative to loam soil, effects of calcareous, clayey and sandy soils on growth of M. oleifera seedlings could be driven by the chemical and physical properties of the test soil types.
The authors have not declared any conflict of interests.
This work was supported by the Agricultural Research Council-Universities Collaboration Centre.
REFERENCES
|
Bezerra AM, Momente E, Medeiros VG, Filho S (2004). Germination of seed and seedling development of drumsticks as a function of seed weight and substrate type. Hortic. Bras. 2:295-299.
Crossref
|
|
|
|
Bouyoucos GJ (1962). Hydrometer method improved for making particle size analysis of soil. Agron. J. 53:464-465.
Crossref
|
|
|
|
|
Brady NC, Weil RR (2009). Elements of the nature and properties of soils. New York, Prentice Hall.
|
|
|
|
|
Dania SO, Akpansubi P, Eghagara OO (2014). Comparative effects of different fertilizer sources on the growth and nutrient content of Moringa (Moringa oleifera) seedlings in a greenhouse trial. Adv. Agric. pp. 1-6.
Crossref
|
|
|
|
|
Food and Agriculture Organization (FAO) (1973). Calcareous soils. Rome, FAO Soils Bulletin 21.
|
|
|
|
|
Foidl N, Makkar HPS, Becker K (2001). The potential of Moringa oleifera for agricultural and industrial uses. In: Fuglie LJ (ed) The Miracle Tree: The Multiple Attributes of Moringa. CTA, USA, pp. 86-90.
|
|
|
|
|
Fuglie JW (2001). The miracle tree: Moringa oleifera, natural nutrition for the tropics.Training Manual. Dakar, Senegal; Church World Service.
|
|
|
|
|
Hegazi MA (2015). Influence of soil type, sowing date and diluted seawater irrigation on seed germination, vegetation and chemical constitution of Moringa oleifera. J. Agric. Sci. 7(3):138-147.
|
|
|
|
|
Hepler PK (2005). Calcium: A central regulator of plant growth and development. Plant Cell.17:2142-2155.
Crossref
|
|
|
|
|
Kabata-Pendias A (2010). Trace elements in soils and plants. Harvard, CRC Press.
Crossref
|
|
|
|
|
Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S (2015). Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 16:12791-12835.
Crossref
|
|
|
|
|
Marschner H (1995). Mineral nutrition of higher plants. London, Academic Press.
|
|
|
|
|
McMahon MJ, Kofranek AM, Rubatzky VE (2005). Hartmann's plant science: Growth, development and utilisation of cultivated plants. Upper Saddle River, Ne Jersey, Prentice Hall.
|
|
|
|
|
Mridha MAU (2015). Prospects of moringa cultivation in Saudi Arabia. J. Appl. Environ. Biol. Sci. 5(3):39-46.
|
|
|
|
|
Obreza TA, Zekri M, Calvert DV (2015). Citrus fertilizer management on calcareous soils. UF/IFAS Extension, CIR1127.
|
|
|
|
|
Pahla I, Tagwira F, Muzemu S, Chitamba S (2013). Effects of soil type and manure level on the establishment and growth of Moringa oleifera. Int. J. Agric. For. 3(6):226-230.
|
|
|
|
|
Ramachanran C, Peter KV, Gopalakrishan PK (1980). Drumstick (Moringa oleifera): A multipurpose tree Indian vegetable. Econ Bot. 34(3):276-283.
Crossref
|
|
|
|
|
Thomas GW. 1996. Soil pH and soil acidity. In: Sparks DL, editor. Methods of soil analysis: Part 3 Chemical methods. SSSA Book Series. Madison, W, Soil Science Society of America.
|
|