ABSTRACT
Tomato (Lycopersicon esculentum Mill.) fruits due to their high moisture content are spoiled and deteriorate in short period of time. Once fruits are harvested, respiration and transpiration are the two major physiological processes that significantly affect storage life and quality of the fruits. However, effects of these processes can be minimized through optimizing harvesting stage of fruits and applying physical barriers for oxygen diffusion and moisture migration. The aim of this work was to investigate the combined effect of stage of harvesting of fruits and application of edible coating materials on storage life and quality of tomato fruits. Treatment combinations were the three harvesting stages of the fruits (mature green, turning and light red stages) and two coating materials (pectin and chitosan with control). Treatments were laid out in a completely randomized design with three replications. Sample fruits were evaluated periodically for different parameters. The study showed that, coating of tomato fruits delayed the ripening process with better fruits quality than uncoated ones. Combined treatment combinations resulted in a significant delay in the change of weight loss, disease incidence, disease severity and ripening index as compared to control fruits. Moreover, in terms chemical parameters, coated fruits revealed higher amount of ascorbic acid, lycopene and phenolic contents. Fruits coated with either chitosan or pectin at turning stage of maturity showed relatively better results for most of the quality parameters. Maximum shelf life was observed for fruits harvested at turning stage coated by pectin (17 days) and chitosan (16 days) films than control (10 days) at the same stage of maturity. Therefore, storage life of the fruits with better quality can be extended by combining optimum stage of harvesting with use of edible coating materials.
Key words: Tomato, harvesting stage, edible coating, pectin, chitosan, storage life.
Tomato is one of the vegetable crops which is widely consumed either raw or after processing and can provide a significant proportion of the total antioxidants in a diet (Martinez-Valverde et al., 2002). Its antioxidants are like vitamin C and E, lycopene, ß-carotene, flavonoids and other phenolic compounds (Dumas et al., 2003). Their activity is based on inhibiting or delaying the oxidation of biomolecules in human body by preventing the initiation or propagation of oxidizing chain reactions (Radzevicius et al., 2009). In addition to this, the fruit also consists of different sugars, acids, phenols and minerals and significant amount of water. However, due to its high moisture content, the fruit is subjected to high rate of metabolic degradation in ambient air. Due to these reasons fast ripening after harvest and softening as well as deterioration during storage is a major problem (Kader, 2008). In tropical countries, about 40 to 50% of post-harvest losses occur between harvesting, transportation and consumption of fresh tomato due to short storage time (Kader, 1992). A study conducted in central rift valley of the country, a postharvest lose of 20.45, 8.63, 2.93 and 7.3% were observed at producers, wholesalers, retailers, hotel and cafeteria levels with a cumulative loss of 39% (Gezai, 2013). Similarly, a study conducted around Dire Dewa region, showed that estimated postharvest losses of tomato was 45.32 (Kasso and Bekel, 2016). This large annual loss of tomato fruits has a great economic and nutritional implication unless and otherwise appropriate ripening control measures are taken to prolong storage life with better quality retention (Hoberichts et al., 2002).
Proper harvesting stage determines the nutrient contents as well as storage durability of any fruit. It was found that maturity stage is an important factor that influences the consumer preferences (Casierra-Posada and Aguilar-Avendaño, 2009). Depending on distance of market, purpose of use and production area, tomatoes can be harvested at different stages of maturity from mature-green stage to full ripe stage. Once harvested, it is recommended to minimize the respiration and transpiration rates of fruits using different methods. Low temperature storage is a recommended method but not feasible for small scale farmers in developing counties. However, in recent years, there is increasing interest to use edible coatings to maintain fruit quality (Tzoumaki et al., 2009). Edible coatings can provide an alternative option to extend postharvest life of fresh fruits with or without low temperature storage. It also has the same effect as modified atmosphere storage or packaging where the internal gas composition is modified (Park, 1999). Edible coating acts as a semi-permeable barrier against O2, CO2, moisture and solute movement, thus reducing respiration rate, water loss and oxidation reaction and then helps to maintain internal quality and appearance (Arvanitoyannis and Gorris, 1999). The use of edible coating has also received more attention in recent years, due to the growing interest for reducing environmental pollution caused by plastics, the need to extend the shelf life of foods, and the increasing demand for healthier and ecological foods (Espino-Díaz et al., 2010).
Pectin is commercially produced from citrus peel as a by-product from extraction of lime, lemon and orange juices; or from apple pomace (
Attila and William, 2009). Under certain circumstances, pectin forms gels; this property has made it a very important as edible coatings. Pectin coatings have been also studied for their ability to retard lipid migration and moisture loss, and to improve appearance and handling of foods (Ayranci and Tunc,
2004).
Chitosan is an edible and biodegradable polymer derived from chitin. Some desirable properties of chitosan are that it forms films without the addition of additives. It has been successfully used in many postharvest aspects of fruit and vegetables (Youwei and Yinzhe, 2013). Therefore, the aim of this work was to determine combined effects of optimum harvesting stage and edible coating material for better fruit quality and extended storage life of tomato fruits.
Experimental site
This experiment was conducted in Jimma University College of Agriculture and Veterinary Medicine (JUCAVM), Department of Post-Harvest Management Laboratory, Jimma, Ethiopia, between May and June, 2014. During the study time, mean temperature and relative humidity inside the laboratory were 22°C±1 and 74.5%±1, respectively.
Experimental material
Tomato fruits ((Lycopersicon esculentum Mill.) fresh type, variety Barbados at different stages of maturity were collected from Jitu Hawassa farm and transported to JUCAVM, Postharvest Management Laboratory. Maximum care was taken to minimize mechanical damage during harvesting, transportation and handling.
Preparation of experimental material
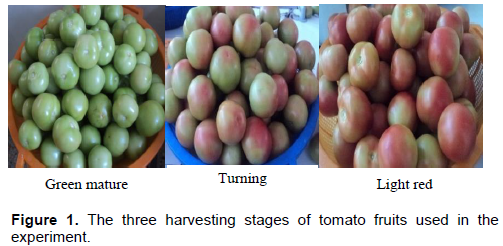
Fruit maturation level was precisely selected and the fruit color was compared in the field using biological color chart of USDA (1991). Harvesting was carried out manually in the morning and uniform shape and size fruits without any injuries or defects were selected. Harvesting stages were mature green (tomato surface is completely green), turning (tannish-yellow, pink or red color showed on over 10%, but not more than 30% of the tomato surface) and light red (pinkish-red or red color showed on over 60%, but red color covers not more than 90% of the tomato surface) (Figure 1). From each stage of maturity for each treatment, 18 uniform sized fruits were washed again with tap water containing 2% (w/v) sodium hypochlorite solution, and rinsed with sterile water, bloated using cheese cloth and left dried at ambient air condition.
Preparation and application of edible coating materials
Preparation of pectin solution
Commercially, available pectin (30 g) with 50% Degree of Esterification was dissolved in 1000 ml warm water (40°C), whilst stirred with magnetic stirrer to prepare 3% (w/v) pectin solution and allowed to homogenized with moderate stirring until the solute completely dissolved, as indicated in Felix and Mahendran (2009).
Preparation of chitosan solution
The chitosan solutions were prepared according to El Ghaouth et al. (1992). An amount of 20 g chitosan was dispersed in 900 ml of distilled water to which 50 ml of glacial acetic acid was added to dissolve the chitosan. The solutions were centrifuged to remove undissolved particles. In order to guarantee the stability of the emulsions, the pH value was adjusted to 5.6 with 1N NaOH. Tween 80 (0.l% v/v) was added to the solutions to improve wettability of the solution during coating.
Application of coating materials
Fruits were uniformly dipped for 2 to 3 min in each solution when the temperature of the solutions reached at room temperature (25°C). Meanwhile control fruits were dipped in distilled water for the same duration and excess water/solution from the fruits were removed and air dried for 3 h at room temperature. A dry layer with plastic texture and general appearance of the fruits were used as criteria to determine the end of surface drying. Surface dried coated fruits were then packed in cardboard boxes with a size of 12 cm L × 10 cm H × 8.5 cm W having 6 openings of each with 7 cm3 size on four sides (except bottom and top parts). The data were recorded before treatment (day 0) and in 5 days interval for all physicochemical parameters for 20 days.
Data collected
Data were collected for both physical, disease and chemical parameters. First, the non distractive parameters were measured then, the distractive measurements were taken.
Physiological weight loss
The weight losses of fruits were recorded on day zero treatment through storage time under ambient storage conditions and then it was recorded in every 5 days intervals. Relative percentage weight loss was calculated using Equation 1 and the cumulative weight loss was expressed as the cumulative percentage for the respective treatments (Athmaselvi et al., 2013).
where WL (%) = percentage physiological weight loss, WI=initial fruit weight in g, and WF=final fruit weight in g at the indicated period.
Fruit ï¬rmness
The method indicated in Fan et al. (1999) was used to determine fruit firmness using Texture Analyzer (TA-XT plus, UK). The force required for the plunger to press into the fruit was recorded and expressed in Newton. Stable Microsystems with 2 mm plunger tip, with flat head stainless-steel cylindrical probe was used to measure tomato fruit firmness. For the current study from each treatment two fruits were used at a time and the average result was used for the analysis. The start of penetration test was the contact of the probe on tomato surface and finished when the probe penetrated the tissues to depth of 5 mm with the probe speed of 1.5 mm/s. The point where the maximum force measured at time of penetration was recorded as the maximum value to determine fruit firmness and expressed in Newton.
Total color change
Total colour change of samples were determined using Commission Internationale de L’Eclairage (CIE) L*a*b* color space to evaluate the effect of coating on color change of samples using tri-stimulus colorimeter (Accu probe HH06), which was calibrated with white tile (L=83.14, a*=-3.67 and b*=10.79). Total color change were expressed in terms of “L*” value, lightness ranging from zero (black) to 100 (white), “a*” (redness) value and “b*” (yellowness) value. Color measurement was made on day zero and when data were collected on specified day intervals. Fruit colors on day zero were considered a target sample color and color changes were evaluated as compared to day zero color. Multiple readings (5 times) per fruit were taken from each sample by changing the position of the tomato fruits to get representative color measurements (Maftoonazad and Ramaswamy, 2005). The total color change (ΔE) was calculated using Equation 2.
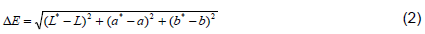
where

is represents the total color change as compared to raw;
L* and
L are initial and final lightness values, respectively;
a* and
a are initial and final redness values, respectively;
b* and
b are initial and final yellowness values, respectively.
Disease incidence
Disease incidence was calculated as number of infested fruits showing any disease symptoms out of the total numbers of tomato fruits stored. Five separate tomato fruits were allocated and used for disease incidence and percent disease index evaluation were
performed according to Hossain et al. (2010) (Equation 3).
Determination of shelf life of stored fruits
The shelf life of tomatoes were calculated by counting the days required for them to attain the last stage of ripening, but up to the stage when they remained still acceptable for commercial marketing. About 10% physiological loss in weight was considered as an index of termination of the shelf life (threshold level) of fruit commodities (Pal et al., 1997; Acedo, 1997).
Determination of pH and titratable acidity (TA)
The fruits were crushed and made into pulp juice, and used to measure the pH using calibrated digital pH meter (CP-505). TA (expressed as % citric acid) was determined by titration (AOAC, 2000). From the juice 5 ml was taken and added in to 250 ml conical flask. Then 10 ml of water was added to make the fruit color light to facilitate clear end point detection. To determine the total TA of the pulp, fresh 0.1 N NaOH was used. TA of tomatoes expressed as percentage of citric acid using Equation 4.
where 1 ml 0.1N NaOH is equivalent to 0.0064 g citric acid.
Determination of TSS
TSS content of tomato fruit pulp was determined using hand held digital refractometer (DR 201-95). The percentage of TSS was obtained from direct reading of the refractometer in °Brix after taking the required temperature correction values. Multiple measurements (3 to 5) were taken per a treatment and the average values were used for analysis.
Determination of TSS/TA ratio (TSS:TA)
The ratio between total soluble solids and titratable acidity was determined by dividing the TSS to that of TA in order to have a sugar-acid balance of samples for each treatment. To calculate the amount of sugar acid ratio, Equation 5 was used:
Determination of ascorbic acid content
Ascorbic acid content was determined by spectrophotometric method (Mohammed et al., 2009). Five grams of tomato sample was mixed with 100 ml of 6% trichloro acetic acid and transferred into a 200 ml volumetric flask and shaken gently to homogenize the solution. The obtained solution was filtered and centrifuged at 4000 rpm for 15 min, and then the sample transferred to a conical flask and 1 to 2 drops of saturated bromine solution was added and aerated, and to each 10 ml aliquot 10 ml of 2% thiourea was added. From 10 ml of aliquot, 4 ml was added into each of test tubes, then 1 ml of 2, 4-DNPH solution was added to form osazone. DNPH reacts with ketone groups of dehydroascorbic acid under acidic conditions to form a red osazone derivative. All samples and blank solution were kept at 37°C for 3 h in a thermostatic hot water bath (WB-8B, China). After all samples were cooled in an ice-water mix for 30 min then treated with 5 ml chilled 85% H2SO4, with constant stirring. Finally, a colored solution absorbance was measured at 521 nm using spectrophotometer (T80 UV/VIS spectrophotometer, UK) and concentration of vitamin C was estimated using Equation 6.
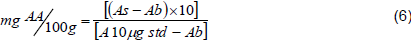
where As = Absorbance of samples; Ab=Absorbance of blank; A10 µg Std=Absorbance of 10 µg AA standard.
Estimation of lycopene content
The lycopene content of the fruits were analyzed according to the method described in Nagata and Yamashita (1992). Briefly, first fruits were crushed and well homogenized, seeds were separated and then one gram of the sample (tomato pulp) was taken. All pigments in the sample were extracted by acetone and hexane (4:6). The samples were well homogenized using homogenizer to extract all pigments in the fruit. After homogenization samples were placed to a beaker and allowed to stand for about 15 min so that there was a clear pigment in a layer of the extractors (acetone and hexane). Finally, the pigments from top part were collected with quartz curvet (10 mm path length) and their absorbance were measured using spectrophotometer’s (T80 UV/VIS, UK) at different wave lengths (663, 645, 505 and 453 nm). Wave lengths measured were used to estimate total lycopene content using Equation 7 as indicated in Nagata and Yamashita (1992):
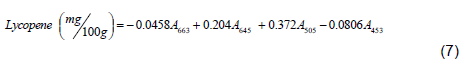
where A663, A505 and A453, are absorbencies at 663, 505 and 453 nm
Determination total phenolic content
Total phenols were measured spectrophotometrically using Folin-Ciocalteu reagent with gallic acid as a standard (Gao et al., 2011). Briefly, 50 μl of tomato extract were added to 3 ml of deionized water plus 250 μl of Folin-Ciocalteu reagent (1N). After a 5 min reaction time, 750 μl of 20% Na2CO3 solution was added. The mixture volume was made up to 5 ml with deionized water. Then, the total phenolic content was measured at 760 nm after a 30 min reaction time using spectrophotometer (T80 UV/VIS, UK). The results are reported in terms of mg of gallic acid equivalent (GAE) per 100 g of fresh weight. Pure Gallic acid (GA) was used as a standard (covering the concentration range between 0.1 and 1.0 mg/ml) (R2 = 0.993) and results were expressed as milligrams of GAE per gram of fresh weight.
Design of the experiment and data analysis
In this study, all the experiments were laid in a Completely Randomized Design (CRD) with a factorial treatment combination, replicated three times, whereby 18 tomato fruits were used per replication.
Significance of treatment effects were evaluated by analysis of variance model using SAS statistical program (Version 9.2) and the mean of the variables whenever significantly different for main or interaction effects, comparisons were made using Tukey’s test at 5% significance level. Data for disease incidence and severity were analyzed using non parametric test.
Physiological weight loss (PWL)
Weight loss is an important index of postharvest storage life in fresh produces. It is mainly attributed to the loss of water during metabolic processes like respiration and transpiration. Both processes are affected by storage environment of the fruit and the loss in weight is an indicator how the product is handled and stored. Because of this, physiological weight loss appeared to be the major detrimental factor of storage life and quality of tomato fruits in particular and horticultural crops in general.
Weight loss of perishable crops has economical implication, a loss in moisture results in loss in weight of product to be sold. In this regard, maturity stage at harvest and coating material showed significant (P<0.05) interaction effect in terms of weight loss reduction as compared to control with extended storage time. For instance pectin and chitosan films coated fruits showed less weight loss in green mature and turning stages than fruits harvested at light red stage (Table 1). This result is in line with Getinet et al. (2008) who reported that the highest weight loss was recorded in Marglobe tomato fruits harvested at light-red stage and the lowest was from Roma VF variety harvested at mature-green stage. However, a significant weight loss was observed from mature green uncoated fruits than coated ones at light red stage.
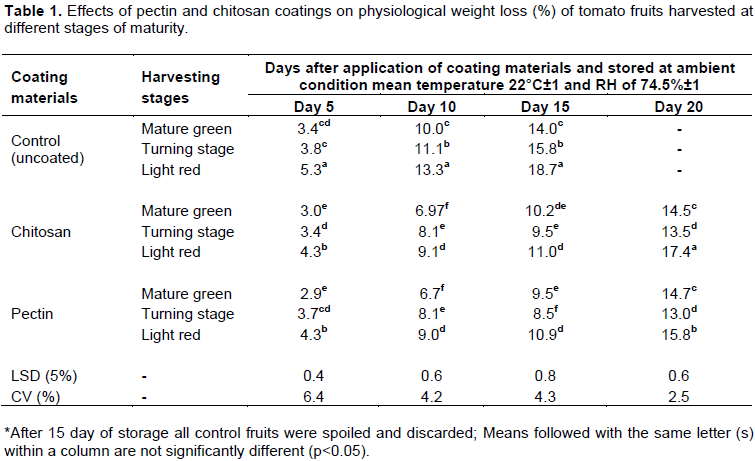
When combined effects are compared, fruits harvested at turning stage and coated with pectin or chitosan film showed lowest loss at 15th and 20th days of storage. But with an increase in storage time, weight loss progressively increased in different rate with the presence or absence of coating films.
Moisture loss and gaseous exchange from fruits is usually controlled by the epidermal layers provided with guard cells and stomata. The film formed on the surface of the fruit act as a physical barrier to reduce moisture migration from the fruits (Togrul and Arslan, 2004). This barrier property also reduces the oxygen availability and uptake by the fruit for respiration process and hence slows down rate of respiration and associated weight (Abbasi et al., 2009).
Fruit firmness
Fruit firmness is a major attribute that dictates the postharvest life and quality of fruits. It is associated to the susceptibility of tomato fruit cell walls to different postharvest handling factors. Firmness of the fruits was better preserved by the application of coatings as seen in Figure 2. The study revealed a significant (P < 0.01) interaction effect between coatings and maturity stages on fruits firmness. Firmnesses of fruits before coating were 9.01, 7.47 and 6.3 N for mature green, turning stage and light red fruits, respectively. The variation is due to the strength of cell wall of fruits at different harvesting stage. However, better firmness values were maintained on coated fruits than uncoated ones (Figure 2). At the end of 15th day storage, uncoated fruits clearly showed the lowest firmness and went to deterioration and discarded. The loss shows that loss in firmness of fruits can be slowed down with the application of coating film, particularly when combined with mature green stage of harvesting as compared to control fruits. In a similar study, Tilahun (2013) showed that the highest values of firmness for mature green fruits than at full ripen stage.
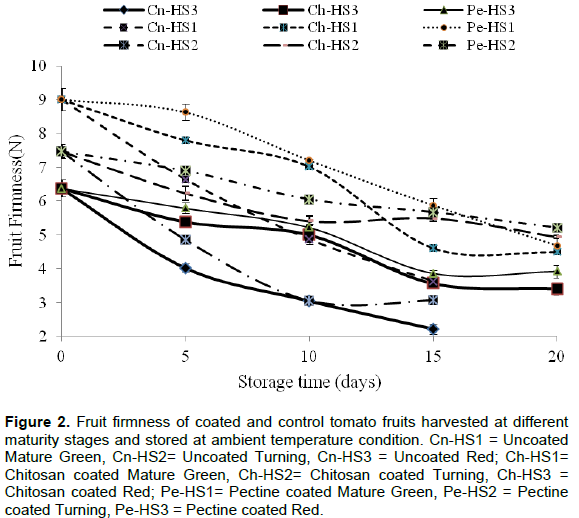
Maftoonazad and Ramaswamy (2008) also indicated that as the length of storage period extended, uncoated peach fruits showed a significant decrease in firmness, while loss of texture and softening were delayed in coated fruits. In their former work, Maftoonazad and Ramaswamy (2005) reported that firmness value in coated samples was almost 1.5 times higher than that of uncoated fruits, as reported for avocados coated with methylcellulose. Similarly, Chauhan et al. (2013) indicated that Shellac based surface coating retained tomatoes’ firmness better than control fruits. Delay in loss of cell wall firmness might be associated with limited availability of oxygen from the ambient atmosphere for respiration process and subsequent delay on cell wall degradation. Generally, the combined treatment effect of coating and early harvesting stage showed beneficial effect on firmness retention as compared to uncoated fruits for distant market shipment. Even though coating materials showed significant interaction effects, but relatively better fruit firmness was observed when pectin coating combined with green mature stage (after 5 and 10 days storage) and turning stage (after 20 days storage). This might be due to storage stability of pectin coating on fruits surface as compared to chitosan film.
Total fruits color change
Color is a very important indicator of ripening and determinant of quality and consumer acceptability. The total color difference (ΔE) extensively used to determine ripening due to chlorophyll degradation and formation of lycopene. It is apparent that tomato fruits harvested at different stages of maturity exhibit color difference.
However, for comparison purpose, original fruit color immediately after coating was taken as a bench mark color to evaluate color changes of fruits. Compared to initial color of fruits, coated fruits showed significant delay on change of color as compared to uncoated ones. Figure 3 shows progressive change of total color change with time from initial values as affected by types of coating materials and maturity stage at harvesting. There was a fast color development from uncoated fruits and become fully turn to red within 2 to 5 days as compared to chitosan and pectin coated fruits (5 to 12 days) depending upon stage of maturity. Similar results were also indicated in Ali et al. (2011), a retardation of color development in papaya fruits coated with higher concentrations of chitosan due to slow rate of respiration and reduced ethylene production. This, in turn, delayed the ripening and senescence of the fruits, resulting in reduced color change. Elevated CO2 levels (>1%) in fruit tissues (which could be achieved by coating materials) might have been shown to retard fruit ripening by inhibiting ethylene synthesis (Martínez-Romero et al., 2007; Zapata et al., 2008).
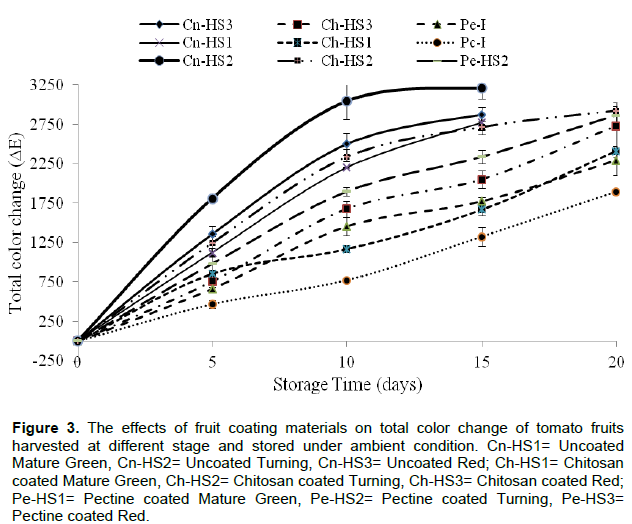
Disease incidence (%)
Results in Table 2 indicate that percent incidence of diseases was significantly (P<0.05) affected by the interaction effect of coating and harvest stages. The incidence was significantly lower on coated tomato fruits as compared with uncoated ones. On the control, fruits harvested at light red stage (more ripen fruits) the first disease occurrence was observed on the 5th day of storage which was 6.7% and as the fruit become ripen they became more susceptible to fungal contamination and exhibited a 100% incidence.
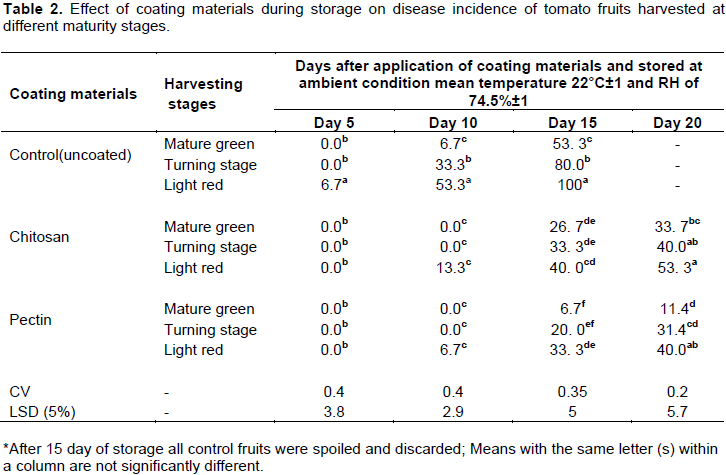
On other hand, after 15th day of storage at ambient conditions the disease incidence on mature green fruits of control, chitosan and pectin coated fruits were 53.33, 26.6 and 6.6%, respectively. Abbasi et al. (2009) also observed that the decay control of irradiated chitosan coated mango fruit as compared to uncoated ones. El-Ghaouth et al. (1991) suggested that chitosan induces chitinase, a defense enzyme, which catalyzes hydrolysis of chitin, a common component of fungal cell walls, thus preventing the growth of fungi on the fruit. Similarly, Zhang et al. (2011) stated that Chitosan could effectively inhibit postharvest diseases of fruits by direct inhibition of spore germination, germ tube elongation and mycelial growth of phytopathogens as well indirect inducement of defense-related enzymes. Antimicrobial capacity of edible coating materials also reported for gum Arabic. Fruits treated with 10% gum arabic coating remained disease free even after 20 days of storage. Many of the control fruits (67%) were spoiled after 16 days of storage (Ali et al., 2010).
As indicated in the earlier sections, application of coating delayed the rate of firmness lose due to preserving cell wall integrity. Furthermore, coating can reduce rate of respiration and ethylene synthesis. These conditions in combination could assist cell wall to retain more integrity against fungal attack (Hassan et al., 2014). Furthermore, coating helps to delay senescence, which makes the commodity more vulnerable to pathogenic infection as a result of loss of cellular or tissue integrity (Tanada-Palmu and Grosso, 2005).
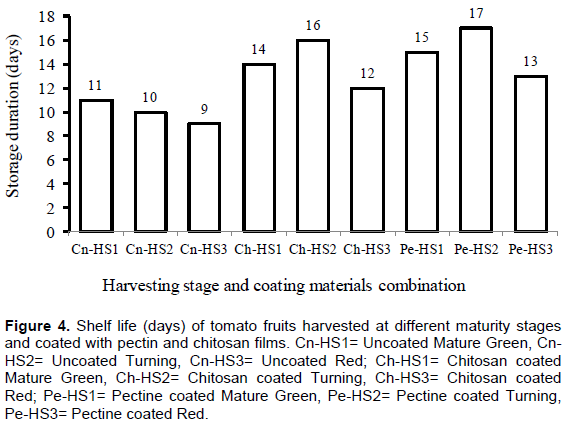
Shelf life (days)
Shelf life implies time period, whereby a product is not only safe to eat, but still has acceptable taste, texture and appearance after being removed from its natural environment (Nieto, 2009). The shelf life of tomato fruits was considerably influenced by the coating and harvesting stages at maturity. In the present study, tomato fruits were decayed within 10 to 20 days of storage after harvesting. As shown in Figure 4, maximum shelf life was observed for tomatoes fruits harvested at turning stage coated by pectin (17 days) and chitosan (16 days) films. However, minimum shelf life was for control tomatoes harvested at light red stage (9 days). For tomatoes harvested at light red stage coated with pectin had a maximum marketable storage life of 13 days followed by chitosan (12 days). Similarly, Maftoonazad and Ramaswamy (2008) also used a pectin-based composite coating on avocados and evaluated the extent of quality changes under different storage temperatures for predicting the quality loss. Their results showed that pectin-based composite coatings significantly reduced the rate of physical, chemical and physiological changes in avocados during storage and extended the storage life by more than a month at 10°C storage. Felix and Mahendran (2009) in their study showed that coated red tomatoes fruits took 15 days to ripe at 30°C, whereas the uncoated ones ripen within 5 days.
pH of fruit pulp
The pH of tomatoes is determined primarily by the acid content of the fruit that determine the product safety. In general, with an increase on days of storage and harvesting stages regardless of coating materials, pH of samples was showed an increase in value. Borji and Jafarpour (2012) and Moneruzzaman et al. (2009) also indicated that the pH of tomato fruit increased with advancement in maturity stage from mature-green to full-ripe stage. Significant (P < 0.05) difference in pH value of tomato fruit was observed due to the interaction effect of maturity stages and coating. The lowest pH values after 15 days of storage were observed for fruits coated with pectin at different stages of harvesting, whereas the highest for uncoated ones (Table 3). A decrease in pH values associated with a decrease in titratable acidity of the fruits and the higher acidity in coated fruits might be because of reduced respiration rate due to limited availability of oxygen (Jiang and Li, 2001). Athmaselvi et al. (2013) also reported that, aloe vera treated tomato fruits were better in keeping pH and showed a better effect in comparison with untreated fruit. The same effect was also reported in Maftoonazad and Ramaswamy (2005), the pH of peache fruits increased at a higher rate in control samples as compared to coated fruits.
Titratable acidity (TA)
The acidity of tomato plays a major role and imparts taste to the fruits. TA is an important consumer variable as the balance of TSS and TA relates to overall taste and consumer acceptability. The TA values of coated and uncoated fruits decreased with storage time (Table 4) and the value was significantly higher (P≤0.05) in chitosan and pectin treated fruits compared to the control due to the interaction effect of maturity stages and coating materials. In coated fruits harvested at turning and mature green stage, TA increased and peaked after 5 days of storage and showed a decline in concentration. Getinet et al. (2008) indicated that higher value in TA (0.67%) in fruits harvested at turning stage and the lowest value (0.58%) was from fruits harvested at mature green stage. On 15th day of storage, the highest TA values were observed for fruits harvested at turning stage but coated with Chitosan and pectin. The values were almost double of that of uncoated fruits harvested at the same maturity stage. This confirms that edible coating materials reduce the rate of acid metabolism (Yaman and Bayoindirli, 2002) as compared to control. Since organic acids, such as malic or citric acid, are primary substrates for respiration, a reduction in acidity is expected in terms of rate of increase in respiration of cells of fruits (El-Anany et al., 2009). The decreasing acidity at the end of storage might be due to use of the acids as energy source with an increase in ripening (Wills et al., 1998; Castro et al., 2005). In another study, Abassi et al. (2009) reported that chitosan coatings slowed the changes on TA of mango, but on control fruits the rate of decline were significantly higher. Result of this study is also in agreement with Ali et al. (2010) who analyzed the effects of gum arabic as an edible coating for preservation of TA in tomato fruit.
Total soluble solids (TSS)
TSS is an important factor to be considered with respect to consumer acceptance. It is expected to increase during ripening and decrease towards senescence (Tasdelen and Bayindirli, 1998). It has been reported that TSS increases with stage of ripeness at harvest (Znidarcic and Pozrl, 2006) and also it generally increases with advancement in maturity during storage (Getinet et al., 2008) which is in agreement with the current result. In the present study, it was observed that a significant (P < 0.05) interaction effect between coating and maturity stages. TSS of control fruits at the end of the storage period (15th day) was 4.8, 4.6, and 4.2 °Brix for fruits harvested at mature green, turning and light red stages, respectively. Borji and Jafarpour (2012) noted that, maturity stages at harvest could affect the TSS content of the fruit. The authors found that the TSS content of mature green and full ripe tomatoes was 5.1 and 6.2 °Brix, respectively. Whereas tomato fruits coated with pectin resulted in 4.9, 5.4 and 4.9 °Brix and that of chitosan coated having a 5.1, 5.5 and 5 °Brix for the same stage of harvesting, respectively. Similar results were also reported when mango fruits were coated with pectin (Moalemiyan et al., 2012). In all cases, fruits harvested at turning stages showed relatively higher TSS values, which might be associated with, higher concentration of organic acid and soluble sugar balance as compared to early or late mature fruits at both stages of harvesting.
Coatings provide an excellent semi-permeable film around the fruit, modifying the internal atmosphere by reducing O2 availability for respiration and degradation of macromolecules. Decreased respiration rates slow down the synthesis and use of metabolites resulting in slower rate of increase on TSS (Yaman and Bayoindirli, 2002). The decrease in TSS is caused by a decline in the amount of carbohydrates and pectins, partial hydrolysis of protein and decomposition of glycosides into sub-units during respiration causing a decrease in TSS (Athmaselvi et al., 2013; Moalemiyan et al., 2012).
TSS/TA ratio as a maturity index
The TSS/TA ratio is an important factor for quality parameters of tomato fruits, since it is known that sweetness and sourness are important criteria for tomato flavour (Stevens et al., 1995). The relationship TSS and TA which could be taken as maturity ripening index (RI) showed a significant differences (P<0.05) as a function of maturity stage, coating and their interaction. The TSS/TA ratio increased significantly along with increased storage time in both uncoated and coated fruits (Table 5). TSS/TA at green stage for control, pectin and chitosan treated fruits at day 5 was 10.09, 8.52, and 7.91, respectively and subsequently reached to 25.65, 17.57, and 19.10 by the end of the storage period with a significant interaction effect between maturity stages and coatings. Generally, coated tomato fruits revealed relatively small ratio changes for all harvesting stages (Table 6). Similar results were also reported in Al-Mughrabi (1994) who demonstrated that harvesting at mature-green stage had lower TSS/TA ratio values in comparison with red-ripe fruits. In general from result of this study, a ratio above 10 can be used as index to determine degree of ripeness of tomato fruits. For instance, uncoated fruit at light red harvesting stage of 5th day of storage showed almost equivalent ratio for coated samples at 15th day of storage. As index of ripening, the ratio can be used to investigate the positive effect of coating materials on preserving of total soluble compounds in fruits as compared to uncoated ones.
Ascorbic acid content
Table 7 shows the changes in the ascorbic acid content of tomato fruits at three maturity stages, treated with chitosan and pectin in 20 days of storage time at ambient temperature. Significant differences were observed
among treatments (P<0.05) for their interaction. For fruits at turning stage, the mean value of ascorbic acid content was 15.80, 34.38 and 38.08 mg/100 g fresh weights for control, chitosan and pectin, respectively (on 15th day after coating). Green mature fruits showed ascorbic acid values of 17.94, 30.60, and 30.10 mg/100 g fresh weight for control, chitosan, and pectin treatments for the same duration of storage. However, light red tomatoes showed ascorbic acid values of 13.03, 22.20, and 26.70 mg/100 g fresh weight for control, chitosan, and pectin treatments (on 15th day after coating). Sharma et al. (1996) reported ascorbic acid content ranged from 11.21 to 53.29 mg/100g in tomato genotypes which is in agreement with values indicated in this study. Similar results were also reported in Tigist et al. (2011) a general trend of increase in ascorbic acid content, followed by a falling during full ripening stage.
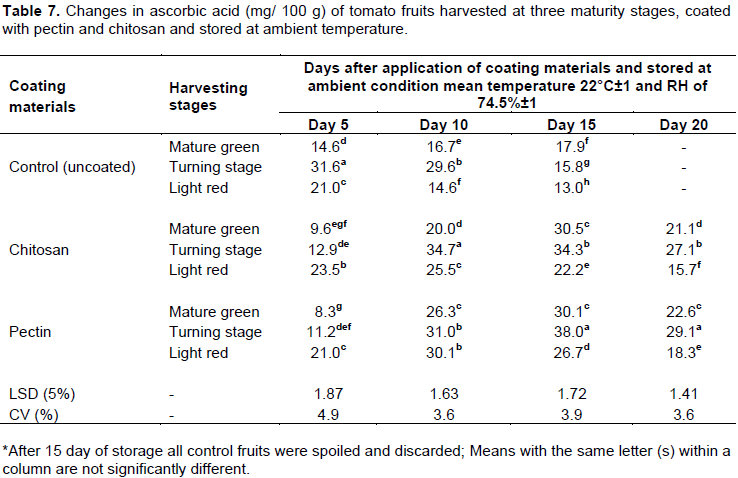
Results illustrated in Table 7 show a reduction in ascorbic acid content along with the storage period not only for coated fruits but also for the control. However, a decrease in ascorbic acid content was significantly higher in control as compared with coated fruits. High ascorbic acid in coated fruits could be attributed with slow ripening rate due to semi-permeable membrane films of chitosan and pectin, since coatings serve as a protective layer and control for the diffusion of O2 (Srinivasa et al., 2006) which is critical to initiate respiration processes (Ayranci and Tunc, 2004). Ali et al. (2010) reported a similar slow down of ascorbic acid degradation for gum Arabic coated tomato during ripening. Likewise Ali et al (2011) also reported papaya fruits coated with chitosan showed a slower initial increase in ascorbic acid as compared to uncoated fruits. This suggests that chitosan and pectin coatings slowed down the synthesis of ascorbic acid during ripening and also slowed down the rate of loss in coated fruits which can be attributed with O2 availability for respiration and oxidation.
Lycopene content
Lycopene is the major carotenoid compound in tomatoes, it gives the fruit its characteristic red color (Frusciante et al., 2007). The lycopene content of tomatoes has been previously reported to be in the range of 0.88 to 4.2 mg/100 g of fresh weight (Clinton, 1999). During ripening, the chlorophyll content decreases, and there is a rapid synthesis of the red pigment lycopene. Table 8 shows the changes in the lycopene content of tomato fruits at three maturity stages coated with chitosan and pectin over 20 days of storage at ambient condition. In the current study, significant (P < 0.05) difference was observed on the lycopene content of tomato fruits due to the interaction effect of maturity stages and edible coating materials.
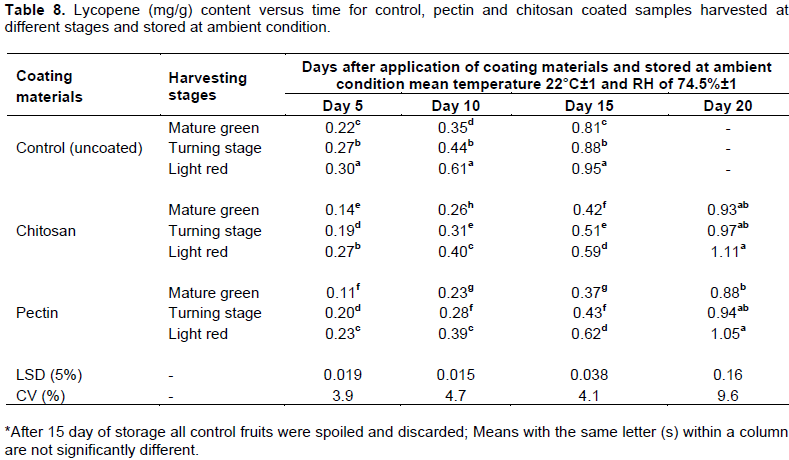
Generally, lycopene content of the tomato fruits increased with the storage time in all treated and untreated fruits (Table 8) which was associated with ripening stages. However, the content of untreated fruits increased sharply and reached to a maximum level after 15 days of storage. But similar lycopene concentration was noted from pectin and chitosan coated fruits on 20th day of storage. The ripening and antioxidant index of the tomatoes (lycopene) also varies from one ripening stage to the other and the variations were also observed with coated and uncoated fruits. The results of the study (Table 8) established that the content of lycopene from all treatments increased with storage time but at different rates. The lowest concentration of lycopene (0.11 mg/100 g) was recorded in pectin coated fruits harvested at green stage after 5 days of storage while the highest concentration of 1.1 mg/100 g for chitosan coated fruits which were harvested at light red stage on 20th day after coating and of storage.
The early increase in lycopene content in control fruits might be due to faster ripening rate of fruits which leads to the conversion of chloroplasts to chromoplasts and lycopene accumulation in internal membrane system (Grierson and Kader, 1986). Results of this study is also in line with Ali et al. (2013) who reported that lycopene content of uncoated tomatoes increased sharply and reached to a maximum peak after 12 days of storage but those coated with gum arabic stayed for 16 days. It has also been reported that the formation of lycopene depends on the rate of respiration during storage (Javanmardi and Kubota, 2006). As indicated in earlier, coatings reduce rate of respiration of fruits through limiting O2 availability. Since uncoated fruits exposed fully to atmospheric oxygen, the lycopene content of red light fruits after 15th days of storage was 0.95, 0.59 and 0.62 mg/100 g, for control, chitosan and pectin coatings.
Total phenolic content
Polyphenols are common constituents of foods of plant origin and are major antioxidants in the human diet. These compounds possess diverse biological properties which provide a number of benefits, including antioxidant, apoptotic, anti aging, anti carcinogenic and anti inflammatory activities, cardiovascular protection, and also inhibit angiogenesis and cell-proliferation (Han et al., 2007). In this study, significant (P < 0.05) difference on total phenolic content of tomato fruit was observed due to the interaction effect of maturity stages and coatings materials. After 10th day of storage, higher values of total phenolic content was observed on fruits harvested at turning stage but coated with pectin (93.2 mg/100 g sample) and followed by chitosan coated fruits (79.6 mg/100 g). The same trend was followed after 15 and 20th days of storage in terms of harvesting stages, but values were decreased when storage time increased to 20th day (Table 9). At this stage of harvesting, fruits could perceive coatings materials as a potential abiotic stress, thereby resulting in production of secondary metabolites like phenols in coated samples (González-Aguilar et al., 2010). The authors indicated that, edible coatings can produce abiotic stress on produce, modifying its metabolism and affecting the production of secondary metabolites such as phenolic and flavonoid compounds due to the oxidative stress created by coating. Previous studies also showed that low O2 and elevated CO2 concentrations increased the production of phenolic compounds during the storage of fresh cut melons, which was related to oxidative stress on the fruit (Frusciante et al., 2007). The accumulation of phenolic compounds may be promoted by PAL enzyme (Phenylalanine ammonia-lyase) activity, which is activated under stress conditions (Wu and Lin, 2002). In grapes treated with edible chitosan coatings, an increase in the PAL enzyme was also observed (Romanazzi et al., 2002).
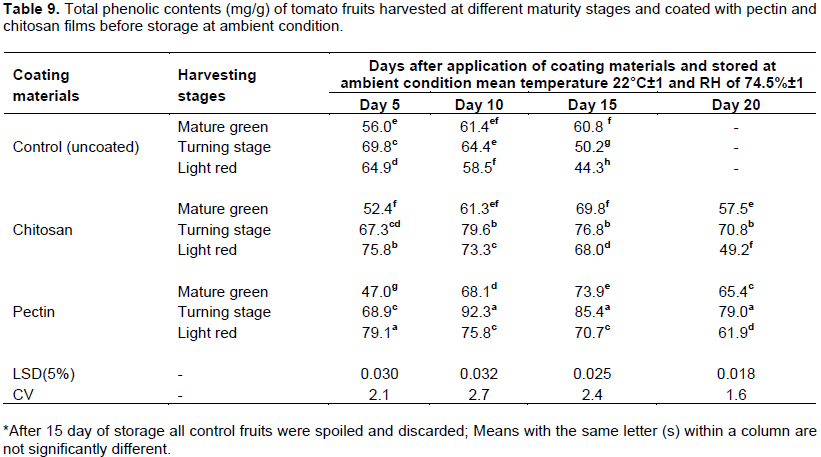
Since phenolic compounds contribute to fruit quality in terms of color, taste, aroma and flavor (Tomás-Barberán and Espín 2001), those coated fruits with higher phenolic content would have higher quality than controls. Furthermore, from health point of view, an increase in total phenolic content is related with the enhancement of antioxidant capacity (Reyes and Cisneros-Zevallos, 2003) of fruits. Similarly, Ali et al. (2010) reported the maximum amount of total phenolic content was observed on gum Arabic coated fruit and reached to a peak after 12 days of storaage and decreased sharply at the final days of storage.
Tomato is a highly perishable fruit that possesses a very short shelf life and reaches to respiration peak of ripening process in short period of time after harvesting. In view of easy adoption and sustainability of technologies, edible coatings can be a good alternative since they are simple, low-cost and environmentally friendly alternative technologies to extend postharvest life and reduce quality loss. The study showed that surface coating of tomato using pectin and chitosan solution can significantly (P< 0.05) delay changes in different quality attributes and the shelf life was extended during ambient storage as compared with uncoated fruits. Maximum shelf life was observed for tomatoes harvested at turning stage coated by pectin (17 days) followed by chitosan (16 days), and minimum shelf life was for uncoated fruits for the same harvesting stage (10 days). The most suitable stage for coating was turning stage for both chitosan and pectin to preserve better quality of tomato fruits. In the present experiment, coated fruits contain higher amount of ascorbic acid, lycopene and phenolic content. As both chitosan and pectin resulted in comparable effect, the choice of type of coating material depends on their price and availability.
The authors have not declared any conflict of interests.
REFERENCES
|
Abbasi NA, Zafar I, Maqbool M, Hafiz IA (2009). Post harvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak. J. Bot. 41(1):343-357.
|
|
|
|
Acedo AL (1997). Storage life of vegetables in simple evaporative coolers. Trop. Sci. 37:169-175.
|
|
|
|
|
Ali A, Mahmud TMM, Sijam K, Siddiqui Y (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 124:620-626.
Crossref
|
|
|
|
|
Ali A, Maqbool M, Alderson PG, Zahid N (2013). Effect of gum arabic as an edible coating on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. Postharv. Biol. Technol. 76:119-124.
Crossref
|
|
|
|
|
Ali A, Maqbool M, Ramachandran S, Alderson PG (2010). Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanumly copersicum L.) fruit. Postharv. Biol. Technol. 58:42-47.
Crossref
|
|
|
|
|
Al-Mughrabi MA (1994). A comparison between postharvest tomato quality of mature green and red-ripe stages produced in hydroponic. J. King Saud Univ. Agric. Sci. 6(2):273-279.
|
|
|
|
|
AOAC (2000). Association of Official Analytical Chemists. Official methods of Analysis 17th ed. of AOAC International. Washington, DC, USA.
|
|
|
|
|
Arvanitoyannis I, Gorris LGM (1999). Edible and biodegradable polymeric materials for food packaging or coating in processing foods. In: Fernanda ARO (Ed.). Quality Optimization and Process Assessment. CRC Press, Florida, USA, pp. 357-371.
|
|
|
|
|
Athmaselvi KA, Sumitha LP, Revathy B (2013). Development of Aloe vera based edible coating for tomato. Int. Agrophys. 27:369-375.
Crossref
|
|
|
|
|
Attila EP, William O (2009). Edible Films and Coatings: Why, What, and How? In: Embuscado ME and Huber KC (eds). Edible Films and Coatings for Food Applications. NY: Springer, pp. 1-23.
|
|
|
|
|
Ayranci E, Tunc S (2004). A method for the measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reactions in fresh foods. Food Chem. 80(3):423-431.
Crossref
|
|
|
|
|
Borji H, Jafarpour GMA (2012). Comparison between tomato quality of mature-green and red- ripe stages in soilless culture. Short Communication. Afr. J. Agric. Res. 7(10):1601-1603.
|
|
|
|
|
Casierra-Posada F, Aguilar-Avenda-o OE (2009). Incidence of Maturity Stage on Tomato (Solanum lycopersicum L.) Fruit Qual. Acta Hortic. 821:229-232.
Crossref
|
|
|
|
|
Castro LR, Vigneault C, Charles MT, Cortez LA (2005). Effect of cooling delay and cold-chain breakage on 'Santa Clara' tomato. J. Food Agric. Environ. 3:49-54.
|
|
|
|
|
Chauhan OP, Nanjappa C, Ashok N, Ravi N, Roopa N, Raju PS (2013). Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 22:1-6
|
|
|
|
|
Clinton S (1999). Lycopene: Chemistry, biology and implication for human health and disease. Nut. Rev. 56:35-51.
Crossref
|
|
|
|
|
Dumas Y, Dadomo M, Di Lucca G, Grolier P (2003). Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 83(5):369-382.
Crossref
|
|
|
|
|
El Ghaouth A, Arul J, Ponnampalam R, Boulet M (1992). Chitosan coating to extend the storage life of tomatoes. Hortic. Sci. 27(9):1016-1018.
|
|
|
|
|
El-Anany AM, Hassan GFA, Rehab Ali FM (2009). Effects of edible coatings on the shelf-life and quality of anna apple (Malusdomestica Borkh) during cold storage. J. Food Technol. 7:5-11.
|
|
|
|
|
El-Ghaouth A, Arul J, Ponnampalam R, Boulet M (1991). Chitosan coating effect on storability and quality of fresh strawberries. J. Food Sci. 56:1618-1631.
Crossref
|
|
|
|
|
Espino-Díaz M, De JesúsOrnelas-Paz J, Martínez-Téllez MA, Santillán C, Barbosa-Cánovas GV, Zamudio-Flores PB, Olivas GI (2010). Development and characterization of edible films based on mucilage of Opuntiaficus-indica (L.). J. Food Sci. 75:347-352.
Crossref
|
|
|
|
|
Fan X, Blankenship SM, Mattheis JP (1999). 1 -Methylcyclopropene inhibits apple ripening. J. Hortic. Sci. 124(6):690-695.
|
|
|
|
|
Felix ED, Mahendran T (2009). Physicochemical properties of mature green tomatoes (Lycopersicon esculentum) coated with pectin during storage and ripening. Trop. Agric. Res. Ext. 12(2):110-111.
|
|
|
|
|
Frusciante LP, Carli MR, Ercolano R, Pernice A, DiMatteo A, Fogliano V, Pellegrini N (2007). Antioxidant nutritional quality of tomato. Mole. Nutr. Food Res. 51:609-617.
Crossref
|
|
|
|
|
Gao H, Cheng N, Zhou J, Wang B, Deng J, Cao W (2011). Antioxidant activities and phenolic compounds of date plum persimmon (Diospyros lotus L.) fruits. J. Food Sci. Technol. 43:1-2.
|
|
|
|
|
Getinet H, Seyoum T, Woldetsadik K (2008). The effect of cultivar, maturity stage and storage environment on quality of tomatoes. J. Food Eng. 87:467-478.
Crossref
|
|
|
|
|
Gezai AW (2013). Assessment on Postharvest Losses of Tomato (Lycopersicon esculentem Mill.) in Selected Districts of East Shewa Zone of Ethiopia Using a Commodity System Analysis Methodology. MSc Thesis Submitted in Partial Fulfillment of the Requirements for Master of Science in Postharvest Management (Specialization: Perishable Produces), Jimma University College of Agriculture and Veterinary Medicine, Jimma, Ethiopia.
|
|
|
|
|
González-Aguilar GA, Ayala-Zavala JF, Olivas IG, de la Rosa LA, Álvarez-Parrilla E (2010). Preserving quality of fresh-cut products using safe technologies. J. Verbr. Lebensm. 5:65-72.
Crossref
|
|
|
|
|
Grierson D, Kader AA (1986). Fruit ripening and quality. In: Atherton JG, Rudich J, eds, The Tomato Crop: A Scientific Basis for Improvement. Chapman and Hall, London, pp. 241-280.
Crossref
|
|
|
|
|
Han X, Shen T, Lou H (2007). "Dietary Polyphenols and their Biological Significance," Int. J. Mole. Sci. 8(9):950-988.
Crossref
|
|
|
|
|
Hassan ZH, Lesmayati S, Qomariah R, Hasbianto A (2014). Effects of wax coating applications and storage temperatures on the quality of tangerine citrus (Citrus reticulata) var. Siam Banjar. Int. Food Res. J. 21:641-648.
|
|
|
|
|
Hoberichts FA, Linus HW, Plas VD, Woltering EJ (2002). Ethylene perception is required for the expression of tomato ripening-related genes and associated physiological changes even at advanced stages of ripening. Postharv. Biol Technol. 26:125-133
Crossref
|
|
|
|
|
Hossain MT, Hossain SMM, Bakr MA, Rahman AKMM, Uddin SN (2010). Survey on major diseases of vegetable and fruit crops in chittagong region. Bangl. J. Agric. Res. 35(3):423-429
Crossref
|
|
|
|
|
Javanmardi J, Kubota C (2006). Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharv. Biol. Technol. 41:151-155.
Crossref
|
|
|
|
|
Jiang Y, Li Y (2001). Effects of Chitosan Coating on Postharvest Life and Quality of Longan Fruit. Food Chem. 73:139-143.
Crossref
|
|
|
|
|
Kader AA (2008). Perspective: flavor quality of fruits and vegetables. J. Sci. Food Agric. 88:1863-1868.
Crossref
|
|
|
|
|
Kasso M, Bekele A (2016). Post-harvest loss and quality deterioration of horticultural crops in Dire Dawa Region, Ethiopia. In press. J. Saudi Soc. Agric. Sci.
Crossref
|
|
|
|
|
Maftoonazad N, Ramaswamy HS (2008). Effect of pectin based coating on the kinetics of quality change associated with stored avocados. J. Food Proc. Preserv. 32(4):621-643.
Crossref
|
|
|
|
|
Maftoonazad N, Ramaswamy HS (2005). Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT - Food Sci. Technol. 38(6):617-624.
|
|
|
|
|
Martínez-Romero D, Bailen G, Serrano M, Guillen F, Valverde JM, Zapata P, Castillo S, Valero D (2007). Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Crit. Rev. Food Sci. Nutr. 47:543-560.
Crossref
|
|
|
|
|
Martinez-Valvercle I, Periage MJ, Provan G, Chesson A (2002). Phenolic compounds, Lycopene and antioxidant activities in commercial varieties of tomato (Lycopersicon esculentum). J. Sc. Food Agric. 82:323-330.
Crossref
|
|
|
|
|
Moalemiyan M, Ramaswamy HS, Maftoonazad N (2012). Pectin-Based Edible Coating for Shelf-Life Extension of Ataulfo Mango. J. Food Proc. Eng. 35:572-600.
Crossref
|
|
|
|
|
Mohammed QY, Hamad WM, Mohammed EK (2009). Spectrophotometric determination of total vitamin C in some fruits and vegetables at Koya Area – Kurdistan Region/ Iraq. Koya Technical Institute Koya College of Science - University of Koya 4:46-51.
|
|
|
|
|
Moneruzzaman KM, Hossain ABMS, Sani W, Saifuddin M, Alinazi M (2009). Effect of Harvesting and Storage Conditions of Postharvest Quality of Tomatoes. Austr. J. Crop Sci. 3(2):113-121.
|
|
|
|
|
Nagata M, Yamashita I (1992). Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Japan. Soc. Food Sci. Technol. 39(10):925-928.
Crossref
|
|
|
|
|
Nieto MB (2009). Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In: Embuscado, ME and Huber KC (eds). Edible Films and Coatings for Food Applications. NY: Springer pp. 57-112.
Crossref
|
|
|
|
|
Pal RK, Roy SK, Srivastava SS (1997). Storage performance of Kinnow mandarins in evaporative cool chamber and ambient conditions. J. Food Sci. Technol. 34(3):200-203.
|
|
|
|
|
Park HJ (1999). Development of advanced edible coatings for fruits. Trends Food Sci. Technol. 10:254-260.
Crossref
|
|
|
|
|
Radzevicius A, KarklelienÄ— R, Viškelis P, Bobinas ÄŒ, BobinaitÄ— R, SakalauskienÄ— S (2009).Tomato (Lycopersicon esculentum Mill.) fruit quality and physiological parameters at different ripening stages of Lithuanian cultivars. Agron. Res. 7(2):712-718.
|
|
|
|
|
Reyes LF, Cisneros-Zevallos L (2003).Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes. J. Agric. Food Chem. 51:5296-5300.
Crossref
|
|
|
|
|
Romanazzi G, Nigro F, Ippolito A, DiVenere D, Salerno M (2002). Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J. Food Sci. 67:1862-1867.
Crossref
|
|
|
|
|
Sharma S, Mahajan R, Bajaj KL (1996). Biochemical evaluation of some tomato varieties. Veg. Sci. 23:7-42.
|
|
|
|
|
Srinivasa PC, Keelara VH, Nuggenahalli SS, Ramasamy R, Tharanathan RN (2006). Storage studies of tomato and bell pepper using eco-friendly films. J. Sci. Food Agric. 86:1216-1224.
Crossref
|
|
|
|
|
Stevens MA, Kader AA, Albright M (1995). Potential for increasing tomato flavor via increased sugar and acid content. J. Am. Soc. Hortic. Sci. 104:40-42.
|
|
|
|
|
Tanada-Palmu PS, Grosso CRF (2005). Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharv. Biol. Technol. 36:199–208.
Crossref
|
|
|
|
|
Tasdelen O, Bayindirli L (1998). Controlled atmospheric storage and edible coating effects on storage life and quality of tomatoes. J. Food Proc. Preserv. 22:303-320.
Crossref
|
|
|
|
|
Tigist M, Workneh T, Woldetsadik K (2011). Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 50:477-486.
Crossref
|
|
|
|
|
Tilahun AT (2013). Analysis of the effect of maturity stage on the postharvest biochemical quality characteristics of tomato (Lycopersicon esculentum Mill.) fruit. Int. Res. J. Pharm. Appl. Sci. 3(5):180-186
|
|
|
|
|
Togrul H, Arslan N (2004). Extending shelf life of peach and pear by using CMC from sugar beet pulp cellulose as hydrophilic polymer in emulsions. Food Hydrocolloids 18:215-226.
Crossref
|
|
|
|
|
Tomás-Barberán FA, Espín JE (2001). Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Agric. Food Chem. 81:853-876.
Crossref
|
|
|
|
|
Tzoumaki MV, Biliaderis CG, Vasilakakis M (2009). Impact of edible coatings and packaging on quality of white asparagus (Asparagus officinalis, L.) during cold storage. Food Chem. 117(1):55-63.
Crossref
|
|
|
|
|
USDA (1991). Standard for Grades of Fresh Tomatoes. United States Department of Agriculture, Agricultural Marketing Service, P. 13.
|
|
|
|
|
Wills RBH. McGlasson B, Graham D, Joyce D (1998). Postharvest: An introduction to the physiology and handling of fruits, vegetables and ornamentals. Univ. New South Wales Press, P 262.
|
|
|
|
|
Wu JY, Lin LD (2002). Ultrasound-induced stress responses of Panax ginseng cells Enzymatic browning and phenolics production. Biotechnol. Progr. 18:862-866.
Crossref
|
|
|
|
|
Yaman O, Bayoindirli L (2002). Effects of an edible coating and cold storage on shelf-life and quality of cherries. Lebnsm. Wiss. Und. Technol. 35:146-150.
Crossref
|
|
|
|
|
Youwei Y, Yinzhe R (2013). Effect of Chitosan Coating on Preserving Character of Post-Harvest Fruit and Vegetable: A Review. J. Food Process Technol. 4(4):8.
|
|
|
|
|
Zapata PJ, Guillén F, Martínez-Romero D, Castillo S, Valero D, Serrano M (2008). Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J. Sci. Food Agric. 88:1287-1293.
Crossref
|
|
|
|
|
Zhang H, Li R, Liu W (2011). Effects of Chitin and Its Derivative Chitosan on Postharvest Decay of Fruits: A Review. Int. J. Mol. Sci. 12:917-934.
Crossref
|
|
|
|
|
Znidarcic D, Pozrl T (2006). Comparative study of quality changes in tomato cv. 'Malike' (Lycopersicon esculentum Mill.) whilst stored at different temperatures. Acta Agric. 87(2):235-243.
|
|