ABSTRACT
In order to evaluate the systemicity of BBTV from one plant of the mat to the physically attached shoots, 60 mats both of “Yangambi Km5”, Musa AAA and those of the false horn plantain “Libanga Likale”, Musa AAB showing severity levels from 0 to 5 were selected in backyards in Kisangani. In addition, 30 sucker corms per genotype were put under macro-propagation and leaf samples of lateral shoots that had emerged were tested using triple antibody sandwich-enzyme linked immuno sorbent assay (TAS-ELISA). In the backyards, for mats with no visible banana bunchy top disease (BBTD) symptoms, none of the analyzed mats with a total of 29 plants of “Yangambi Km5” and of 35 plants of “Libanga Likale” tested ELISA positive, indicating the absence of the BBTV infection. However, for the severity levels of one to five, 32 to 63.5% of plants in the mats were ELISA positive for “Yangambi Km5”, while 34.9 to 73.2% of plants from “Libanga Likale” tested positive for BBTV. After macro-propagation, 100% of lateral shoots of both cultivars at BBTD severity levels 4 and 5 tested positive. On the other hand, none of the lateral shoots at level 0 tested ELISA positive. However, for levels 1 to 3 some ELISA negative plantlets (40 to 23% for “Yangambi Km5” and 53 to 15% for “Libanga Likale”) were observed. This study indicates the need for the complete destruction of all mats harbouring plants with BBTD severity levels of 3, 4 and 5. Macro-propagation of suckers with severity level 1 symptoms could produce virus-free plantlets but ELISA testing of the lateral shoots is essential to pinpoint the virus-free plantlets.
Key words: Banana bunchy top viral infection (BBTV), macro-propagation, mat, systemicity, triple antibody sandwich-enzyme linked immuno sorbent assay (TAS-ELISA).
Banana bunchy top disease (BBTD) caused by the banana bunchy top virus (BBTV) is one of the most damaging banana diseases in affected tropical regions of Africa, Asia and the Pacific. Potential yield losses of 90 to 100%, especially with ‘Cavendish’ subgroup of the AAA cultivar group (AAA), have been reported in both small-scale farms and in large commercial plantations (Moffat, 2001). The history of the spread of the disease in Africa has been described by Blomme et al. (2013).
Currently, the impact of BBTD has been felt in 15 African countries: Egypt (first recorded in 1901), the Democratic Republic of Congo (DR Congo) (1958), Eritrea (1964), Gabon, Congo-Brazzaville and Equatorial Guinea (1982), Burundi and Rwanda (1987), Malawi, Angola, Cameroon, Central African Republic and Zambia (1990), Benin (before 2011), and Nigeria (2012) (Fahmy, 1927; Wardlaw, 1961; Saverio, 1964; Fouré and Manser, 1982; Sebasigari and Stover, 1988; Pillay et al., 2005; Kumar and Hanna, 2008; Kumar et al., 2011; Blomme et al., 2013; Kumar et al., 2015). In the DR Congo, BBTD has been reported in all 11 provinces (Kumar et al., 2011; Ngama et al., 2014). In DR Congo, BBTD was first identified in the 1950s at the Institut National pour l’Etude et la Recherche Agronomique du Congo Belge (INEAC), Yangambi research station (Kumar et al., 2011) and has since spread to all 11 provinces (Ngama et al., 2014; Mukwa et al., 2014). Disease severity is however low, and only a minority of mats (10%) exhibit the severity levels 4 and 5 characterized by the typical bunchy top aspect of the plant (Ngama et al., 2014). In eastern DR Congo and the Congo basin, the disease seems not to affect mats which includes the fruit-bearing mother plant, its suckers and the underground rhizome in a rapid and systemic way, though one lateral shoot after another do get affected in diseased mats (Walangululu et al., 2010).
Generally, viral diseases are considered systemic, except in the meristematic apex tissues which can be, according to species, free of virus (Thomas et al., 1994). BBTV can be transmitted through the use of vegetative planting material including suckers and in vitro-derived plantlets. Generally, when a parent plant is infected, it is considered that all the physically attached suckers (that is, lateral shoots) will be infected (Gregory et al., 1995). The infections result in a range of symptoms, starting with streaks on the leaf lamina, petioles and midribs, progressing to partial leaf chlorosis, leaf dwarfing and necrosis (Caruana, 2003). Precise identification of the disease at the initial stages (that is, streaks or slight to be backed up with an immuno-enzymological test triple discolorations on the leaves) is often difficult and needs antibody sandwich-enzyme linked immuno sorbent assay (TAS-ELISA) (Hu et al., 2007). It is therefore recommended to destroy the entire banana mat when one plant on it shows BBTD symptoms at any level of severity (Ferreira et al., 1989; Thomas and Dietzgen, 1991). However, very few scientific papers or reports describing BBTV systemicity, are currently available.
The aim of this study was to assess the systemicity of the transmission of BBTV from parent plants to physically attached lateral shoots, taking into account various initial disease severity levels of the parent plant, to elucidate the level of systemicity in banana mats, and to verify if some of the attached lateral shoots could possibly escape the virus. The results of these studies could guide control strategies for fighting BBTD in a region where people find it difficult to destroy a complete mat (and often very large mats) when only one or a few plants are visibly infected.
This study was conducted in Kisangani, Oriental Province, DR Congo. The city is located near the Equator and experiences a continental equatorial climate of Köppen Af classification (Bultot, 1950, 1977). The mean temperature is relatively high (23.5 to 25.3°C) and the mean annual precipitation is about 1,728 mm, with a minimum of 1,417 mm and a maximum of 1,975 mm. Relative humidity is about 82% (www.accuweather.com). The studies were conducted on diseased mats grown in backyards in Kisangani town and using infected corms which were put into macro propagation. The city is entirely located in the bioclimatic zone of ombrophile dense forest. The experimental site was located at an altitude of 409 m above sea level, at latitude 0°30’41.4’’ N and longitude 25°12’24.2’’ E. The study was conducted from September, 2013 to September, 2014.
Unmanaged mats (that is, a cluster of physically interconnected/attached plants) can have a very large number of plants, comprising fruit baring plants, flowering plants and plants at various stages of vegetative development. For example, from 10 to 20 plants can be counted on un-managed mats of the ‘Yangambi Km5’ cultivar. Each larger plant in a mat will have one or more lateral shoots.
To evaluate the systemicity of BBTV from one plant of the mat to the physically attached shoots, 60 mats (30 mats of ‘Yangambi Km5’, Musa AAA and 30 mats of the False Horn plantain ‘Libanga Likale’, Musa AAB cultivars) comprising a total of 530 plants, showing severity levels from 0 to 5, were selected in backyards in Kisangani town (Table 1) (level 0: no symptoms, 1: dark green streaks on the leaf lamina, 2: dark green streaks on the leaf midribs and petiole, 3: marginal chlorosis of the leaf margin, 4: reduction in leaf size/dwarfing of leaves and 5: bunchy top appearance). Visual observations were made on all plants per mat to determine the highest severity level of the disease in the population of plants on a mat and the severity level of the other plants in the mat. For instance, a mat containing a plant with highest severity level 5 could bear plants with levels 4, 3, 2, 1 and 0, while a mat containing a plant with highest severity level 4 could bear plants with severity levels 3, 2, 1 and 0. The immuno-enzymological status of all plants in a mat was then tested using TAS-ELISA.
In addition, five sucker corms for each of the two cultivars (as aforementioned) and for each of the BBTD severity levels 0, 1, 2, 3, 4 and 5 were put in macro-propagation in a screen house after removal of their apical meristem. The screen house was devoid of aphids. Before screen house establishment, all suckers were tested using TAS-ELISA and were confirmed as positive, except for the 0 level suckers where ELISA results were negative. A total of 30 suckers were thus used for each genotype. All plants were allowed to grow until progenies (lateral shoots) had developed at least one expanded leaf. Samples from the expanded leaf were used to assess the presence of BBTV in the lateral shoots using the TAS-ELISA AgdiaBioford ELISA reagent kit. A total of 216 leaf samples were analyzed (Table 3). The TAS-ELISA method used involved BBTV extraction from the leaves, incubation and addition of monoclonal antibody and antibody coupled to alkaline phosphatase B in the presence of positive and negative BBTV controls (Sastry et al., 1980; Soweha, 2005).
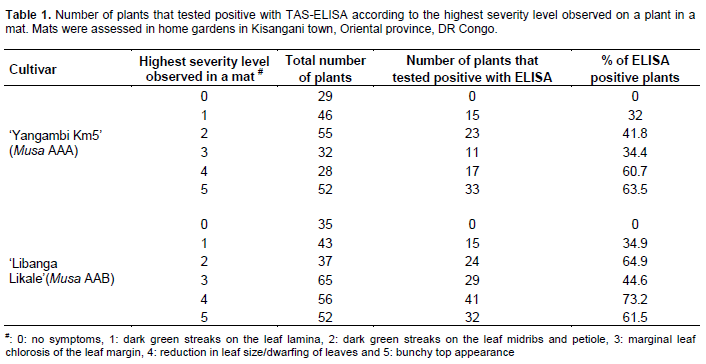
For mats assessed in the backyards,
none of 29 plants analyzed of the 242 plants in the 30 mats of ‘Yangambi Km5’ and 35 plants of the 288 plants from 30 mats of ‘Libanga Likale’ with no visible BBTD symptoms tested ELISA positive, indicating the absence of BBTV infection (Table 1). However, for the severity levels one to five, 32 to 63.5% of plants in the mats were ELISA positive for ‘Yangambi Km5’, while 34.9 to 73.2% of plants tested positive for ‘Libanga Likale’. When looking at plants with similar severity levels across all assessed mats, for severity levels one to five, 100% of plants of ‘Yangambi Km5’ with BBTD symptoms tested positive for BBTV infection, while none of the 143 plants without BBTD symptoms tested positive (Table 2). For ‘Libanga Likale’, the situation was a little different, with some variations observed for level 1 (54% ELISA positive plants) and level 3 (86% ELISA positive plants). Plants of other severity levels (2, 4 and 5) had 100% positive scores, while none the 100 symptomless plants tested positive.
After macro-propagation 100% of lateral shoots derived from parent plants of both cultivars, at BBTD severity levels 4 and 5, tested positive (Table 3). On the other hand, and for both cultivars, none of the lateral shoots at level 0 tested ELISA positive. However, for levels one to three some ELISA negative plantlets (23 to 40% for ‘Yangambi km5’ and 15 to 53% for ‘Libanga Likale’) were observed. There was a clear positive relationship between the BBTD severity level of the parent plant and the proportion of lateral shoots showing positive ELISA tests (Figure 1).
In the home backyard gardens, a tendency for a higher percentage of BBTV infected plants was observed with an increase in highest severity level observed within a mat. These results hint at a systemic transmission in situ, especially visible at higher BBTD severity levels, although some transmission could have occurred via aphids.
Concerning macro-propagation, all the lateral shoots of ELISA positive parent plants of both genotypes at severity levels four and five were infected, indicating a truly systemic infection. Few lateral shoots from severity level 1 to3 (23 to 40% for ‘Yangambi km5’ and 15 to 53% for ‘Libanga Likale’) were ELISA negative, that is, virus free. It is however very clear that the infection was systemic (as the trial was conducted in the absence of aphids) and the few remaining clean suckers, if left on the mother plants would possibly also become infected in a systemic way.
The results presented here have important implications: the need for the complete destruction of all mats harbouring plants expressing disease severity levels 3, 4 and 5
. However, for mats containing only a few plants that show severity levels 1 or 2 (mild symptoms that have not been reported as affecting plant growth or yield), an option could be to only remove these mats if or when more advanced symptoms appear. It was observed that plants with severity levels 1 or 2 still produce harvestable bunches. Macro-propagation of suckers with severity level 1 symptoms could be envisaged for the production of virus-free plantlets as 40 to 50% of lateral shoots on these corms were temporarily observed to be virus free. TAS-ELISA testing of the lateral shoots would however be required to identify the virus-free plantlets.
The results from both the backyard and macro-propagation studies strongly hint at a complete systemic movement of the BBTV and are in accordance with reports from Magee (1927), Ferreira et al. (1989), Thomas and Dietzgen (1991) and Gregory et al. (1995).
The authors have not declared any conflict of interest
REFERENCES
|
Blomme G, Ploetz R, Jones DR, De Langhe E, Price N, Gold C, Geering A, Viljoen A, Karamura D, Pillay M, Tinzaara W, Teycheney PY, Lepoint P, Karamura E, Buddenhagen I (2013). A historical overview of the appearance and spread of Musa pests and pathogens on the African continent: highlighting the importance of clean Musa planting materials and quarantine measures. Ann. Appl. Biol. 162:4-26.
Crossref
|
|
|
|
Bultot F (1950). Carte des régions climatiques du Congo belge établie d'après les critères de Köppen, Publications de l'INEAC, Bruxelles, 13p.
|
|
|
|
|
Bultot F (1977). Atlas climatique du bassin Zaïrois. IVème Partie:
|
|
|
|
|
Caruana IML (2003). Analyse du risque phytosanitaire (ARP) de bananier. Banana Bunchy Top Babuvirus. Ministère de l'agriculture Gouvernement Français/IMG /BAN-c4.X. Mourichon / CIRAD. 31p.
|
|
|
|
|
Fahmy T (1927). Plant diseases of Egypt.Minerals and agriculture in Egypt. Bulletin P 30.
|
|
|
|
|
Ferreira SA, Trujillo EE, Ogata DY (1989). Bunchy Top Disease of Bananas Commodity Fact Sheet.College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa.
|
|
|
|
|
Fouré E, Manser PD (1982). Note sur l'apparition au Gabon d'une grave maladie virale des bananiers et plantains: le Bunchy Top. Fruits 37(6):409-414.
|
|
|
|
|
Gregory JH, Robert MH, James LD (1995). Movement and transmission of banana bunchy top virus DNA component on in bananas. J. Gen. Virol. 76:2279-2285.
Crossref
|
|
|
|
|
Hu JM, Fu HC, Lin CH, Su HJ, Yeh HH (2007). Re assortment and concerted evolution in Banana bunchy top virus genomes. J. Virol. 81:1746-1761.
Crossref
|
|
|
|
|
Kumar PL, Selvarajan R, Iskra CML, Chabannes M, Hanna R (2015). Control of Plant Virus Diseases Vegetatively-Propagated Crops. In: Gad L and Nikolaos IK. New-York: Academic Press. Adv. Virus Res. pp. 229-269.
|
|
|
|
|
Kumar PL, Hanna R (2008). Banana bunchy top virus in sub-Saharan Africa: established or emerging problem? Poster presentation 'Banana and Plantain in Africa: Harnessing international partnerships to increase research impact, workshop held, October 5-9, Mombasa Leisure Lodge Resort, Kenya.
|
|
|
|
|
Kumar PL, Hanna R, Alabi OJ, Soko MM, Oben TT, Vangu GHP, Naidu RA (2011). Banana bunchy top virus in sub-Saharan Africa: investigations on virus distribution and diversity. Virus Res. 159:171-182.
Crossref
|
|
|
|
|
Magee CJ (1927). Investigation on the bunchy top disease of the banana. Council Sci. Ind. Res. Bull. Melbourne. 30:1-64.
|
|
|
|
|
Moffat AS (2001). Finding new ways to fight plant diseases. Science 292:2270-2273.
Crossref
|
|
|
|
|
Mukwa FTL, Muengula M, Zinga I, Kalonji A, Caruana IML, Bragard C (2014). Occurrence and Distribution of Banana Bunchy Top virus Related Agro-ecosystem in south western Democratic Republic of Congo. Am. J. Plant Sci. 5:647-568.
Crossref
|
|
|
|
|
Ngama BJF, Ibanda NB, Komoy LJ, Lebisabo BC, Muhindo SH, Walunkonka BF, Wembonyama LJ, Dhed'a DB, Lepoint P, Sivirihauma C, Blomme G (2014). Assessing incidence, development and distribution of banana bunchy top disease across the main plantain and banana growing regions of the Democratic Republic of Congo. Afr. J. Agric. Res. 9(34):2611-2623.
Crossref
|
|
|
|
|
Pillay M, Blomme G, Rodrigues E, Ferreira AL (2005). Presence of banana bunchy top virus in Angola. Info. Musa 14:44-45.
|
|
|
|
|
Sastry KS, Rao DG, Singh SJ (1980). Studies on control of bunchy top of banana. In: National Seminar on Banana Production Technology. Tamil Nadu Agric. Univ. pp. 144-146.
|
|
|
|
|
Saverio B (1964). Banana cultivation in Eritrea and its problems. Edagricole 8:5-56.
|
|
|
|
|
Sebasigari K, Stover RH (1988). Banana Diseases and Pests in East Africa.Report of a survey in November 1987. INIBAP, Montpellier, France. pp. 3-7.
|
|
|
|
|
Soweha HE (2005). Serological and sero-molecular studies on banana bunchy top disease and in different parts of virus-infected banana plants. J. Agric. Soc. Sci. 1(3):273-275.
|
|
|
|
|
Thomas JE, Caruana MLI, Jones DR (1994). Banana Bunchy Top Disease. Musa Disease Fact Sheet No. 4. International Network for the improvement of Banana and Plantain, Montpellier, France.
|
|
|
|
|
Thomas JE, Dietzgen RG (1991). Purification, characterization and serological detection of virus-like particles associated with banana bunchy top disease in Australia. J. Gen. Virol. 72:217-224.
Crossref
|
|
|
|
|
Walangululu MJ, Matara MR, Bahati L, Niyongere C, Lepoivre P, Blomme G (2010). Assessing the spread and seasonal influence of fruit peel disease and banana bunchy top disease in South Kivu, eastern DR Congo. Tree For. Sci. Biotechnol. 4(2):98-104.
|
|
|
|
|
Wardlaw CW (1961). The Virus Diseases: Bunchy Top. Banana Diseases, including Plantains and Abaca. London: Longmans. pp. 68-115.
|
|