ABSTRACT
Sugarcane (Saccharum spp) is an expanding culture for the production of bioethanol around the world which requires certain practices to improve its productive performance at the different ecosystems. This work's aim is to evaluate the initial Sugarcane growth and drought tolerance through the application of biostimulants. For this purpose an experiment was conducted in a greenhouse using completely randomized design. Prior to planting, the cuttings were treated with: T1 - Water; T2 - Indolebutyric Acid (IBA); T3 - Boron + Zinc; T4 - Tryptophan; T5 - Kymon Plus® + Potamol®; and T6 - Stimulate®. Morphological analysis was performed at 40 and 124 days after planting (DAP) to assess the plant initial growth. The biostimulants effect on drought stress mitigation was evaluated at 120 DAP, after 3 days of suppression irrigation. The IBA and Stimulate® application delivered higher growth rates and biomass accumulation. When compared to Control treatment, the application of Ubyfol® and Stimulate® provided higher photosynthesis in the absence of drought stress and had higher PSII effective quantum yield even when plants were under drought stress. The application of Stimulate® enabled plants to maintain higher photosynthetic, transpiration and stomatal conductance rates under moderate drought stress.
Key words: Drought stress, photosynthesis, Saccharum spp, transpiration
Sugarcane (Saccharum spp.) culture has great economic, social and environmental importance due to its large planted areas and capability to generate the raw material source for sugar and ethanol agribusiness (Ferreira Júnior et al., 2012). The productive performance of sugarcane is dependent on several factors, including the use of growth regulators (Serciloto, 2002; Silva, 2010; Ayele et al., 2014). Recent years show an increase in the use of products known as plant biostimulants for a higher crop yield. It is already considered a common technique in crops such as Rice (Garcia et al., 2009), Cotton (Albrecht et al., 2009), Soybean (Bertolin et al., 2010), Corn (Santos et al., 2013) and Sugarcane (Serciloto, 2002; Ayele et al., 2014). These substances used both in-furrow and as foliar application in sugarcane, have increased yield from 6% to 21%, with response magnitude non-dependent neither from cultivars nor from planting environments (Silva, 2010; Silva et al., 2010).
The growth regulators or bioregulators have broad applicability in numerous crops and are similar to plant hormone substances (Albrecht et al., 2011). Biostimulants can be found within the regulators category, these are mixtures of one or more bioregulators with compounds of different chemical nature such as: amino acids, enzymes, vitamins, minerals, etc. (Castro, 2006). Its definition is still evolving due to the biostimulators concept amplitude, however the European Biostimulants Industry Council (EBIC) defines it as a “substance(s) and/or micro-organisms whose function, when applied to plants or rhizosphere, is to stimulate natural processes to enhance nutrients absorption, nutrient efficiency, tolerance to abiotic stress and crop quality” (Calvo et al., 2014).
Recent studies show that the use of Stimulate® promotes greater dry matter accumulation on plant aerial parts (Garcia et al., 2009) of rice, as well as an increase in yield of ratoon sugarcane (Silva et al., 2010), and as such, a potentiator of crop performance. However no test has yet been performed to confirm if it would raise the sugarcane tolerance to drought conditions. Other root-promoting substances, such as IBA, a synthetic auxin, are widely used to improve rooting of cuttings of several species, especially for those with difficulty in rooting (Fachinello and Kersten, 1996).
Mineral nutrients, such as zinc and boron, have marked influence on tryptophan synthesis and on the transport of indole acetic acid (Goldbach et al., 2001). Tryptophan is an amino acid biosynthetic precursor of several indole substances, such as indole acetic acid (Haggquist et al., 1988). For example, in Zn-deficient bean (Phaseolus vulgaris), the level of indole acetic acid on the shoot tips and young leaves decreased to about 50% (Cakmak et al., 1989). In the same way, boron stimulated root growth in Vicia Fava L. (Liu et al., 2000) and in barley (Choi et al., 2007).
Water deficit has a strong importance in several aspects of plant growth; the most apparent effects are plant size, leaf area and crop yield reduction (Kramer, 1983). Artlip and Wisniewski (2002) divide the drought responses in four types: 1) growth limitation; 2) water loss minimization; 3) morphological adaptations; and 4) physiological adaptations. Amongst the factors influencing plants growth and development, the chemical signals, such as the hormones synthesized by the plant, were some of the most relevant.
Based on the hypothesis that biostimulants and root-promoting substances provide greater sugarcane growth during the tillering phase, even when under drought conditions, this work’s aim was to evaluate the sugarcane’s initial growth and drought stress tolerance under these products’ influence.
The experiment was conducted at Agrarian Sciences Center at the Federal University of Alagoas – Brazil, in a greenhouse at the geodetic coordinates 09°28'02'' S; 35°49'43" W and 127 m altitude, during December 2010 and April 2011.
Before the experiment was setup, a soil chemical and physical analysis was performed (Table 1). The soil used in the experiment was air dried, disaggregated and sieved. The experiment was conducted in 20 L polyethylene pots containing 25 kg of soil. The sugarcane variety used was the RB92579.
The experiment was conducted in a completely randomized design comprising four replicas to evaluate the initial growth and drought stress effect and eight replicas for final growth evaluation. After 3 days suppressing irrigation, at 117 DAP, water deficit was imposed until leaf wilting was visible. Initial growth was evaluated 40 days after planting (DAP). Final growth was evaluated at 124 DAP.
The chemical treatments were as follows: T1 – Control (water); T2 - Indolebutyric Acid (IBA) at 1000 mg L-1; T3 – Boron + Zinc (Borax at 10 kg ha-1 + Zinc Sulfate at 20 kg hectare-1); T4 – Tryptophan at 7.2 kg ha-1; T5 - Kymon Plus® at 1.0 L ha-1 + Potamol® at 0.5 L ha-1 and T6 - Stimulate® at 0.5 L ha-1. Stimulate® has 90 mg L-1 of Kinetin, 50 mg L-1 of Gibberellic Acid and 50 mg L-1 of 4-Indol-3-ylbutyric Acid, Kymon Plus® already has 9.0% of N + 3.0% of K2O + 11.5% of Organic Carbon + contains Polihexose and Potamol® and has 14.0% of Mo, in Potassium Molybdate form + 10.0% de K20 + Polihexose. Commercial recommendations were used for product application based on estimating pot area and soil amount in the pots, considering a soil depth of 0.2 m and soil density of 1200 kg m-3.
The products were diluted in water and sprayed onto the plant cuttings using micro sprinklers, with the exception of the IBA treatment, where cuttings were immersed for ten seconds in the IBA solution and planted just after. The IBA was dissolved in a small amount of NaOH 1 N solution, before being mixed with water.
After plantlets emergence, thinning was performed leaving only two plantlets per pot. The plants were kept under irrigation close to the field capacity up to 117 DAP with water content of 0.17 m3.m-3 V/V, measured with a soil moisture sensor, model SM200, connected to a HH2 moisture meter (DELTA-T Devices, Ltd., Cambridge, England). The irrigation was then suspended for 3 days to induce drought stress, when water content reached 0.015 m3.m-3, with visible leaves wilting on some of plants. At 120 DAP physiological analyzes were performed.
Environmental conditions were monitored during the experiment by an automatic weather station model WS-GP1 (DELTA-T Devices, Cambridge, England), located inside the greenhouse, recording temperature and relative humidity every 5 min and Solar radiation every 10 s. Based on the temperature and humidity data the vapor pressure deficit (VPD) was calculated. Environmental conditions during physiological analysis are show in Figure 1.
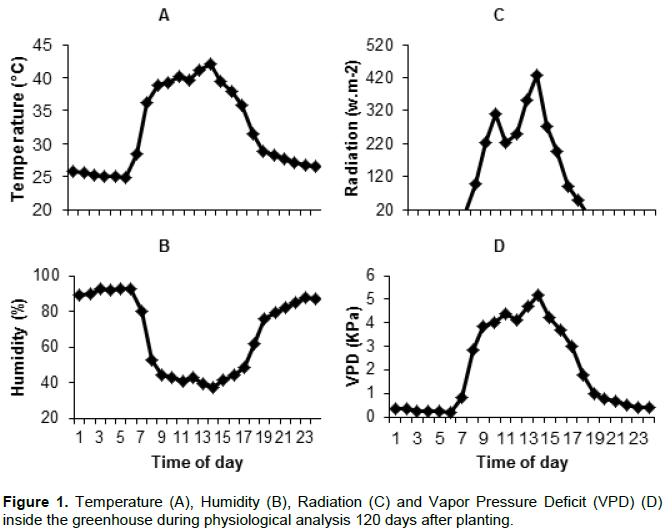
Two biometric evaluations were conducted, the first at 40 DAP and the second at 124 DAP. On both evaluations the following was measured: plant height (cm), number of fully expanded leaves (with at least 20% greenness), number of tillers per pot, length (cm) and width (cm) of the +3 leaf (third leaf bellow the last exposed dewlap). The leaf area (cm²) was quantified by the method described by estimating the area of individual leaves from measurement of leaf width and length (Sinclair et al., 2004). The plants were then collected, separated into leaves, culms and roots, and dried in a kiln with forced air circulation at 70°C until a constant weight was reached to obtain the dry mass (DM).
Based on morphological data from the first (40 DAP) and second (124 DAP) evaluations, a quantitative growth analysis was performed as per below (Benincasa, 1988; Cairo et al., 2008):
At 120 DAP, after inducing drought stress for 3 days, with visible leaf wilting, physiological analyzes were performed only on plants treated with the commercial products to see if they would induce drought stress tolerance. Whilst the SPAD index was quantified in all treatments.
The photochemical efficiency was measured on the middle third of the top visible dewlap (TVD) leaves of each plant at 120 DAP. The maximum quantum yield (Fv/Fm) was measured at 4:30am and at noon, with a portable chlorophyll fluorometer, model OS-1FL (ADC BioScientific, Ltd., Hoddesdon, England), with saturating modulated light pulse of 1s duration, according to the method described by Maxwell and Johnson (2000). The Fv/Fm was measured after dark conditioning, with plastic tweezers for 20 minutes. The PSII effective quantum yield (ΦPSII) was measured between 11:00am and noon on the same leaves taken to measure Fv/Fm, performing two readings per plant, according to Schreiber et al. (1995).
The chlorophyll content on the sugarcane leaves was estimated in vivo via SPAD index with a portable chlorophyll meter, model SPAD-502 (Minolta Co, Ltd., Osaka, Japan), at 122 DAP. Eight random readings were performed on the middle third of the TVD leaf of each plant.
Gas exchange quantification was performed with a portable photosynthesis system (IRGA), model LCi (ADC BioScientific, Ltd., Hoddesdon, England) in the middle third of the TVD leaves between 10:00am and noon at 120 DAP, with one reading per plant. All measurements were made at ambient CO2 concentration and humidity. Short term fluctuations of CO2 and humidity were eliminated by sucking atmospheric air into a 20 L plastic chamber prior to the equipment measurements being taken. The CO2 concentration in the cuvette was stable close to 365 µL L-1. The photosynthetic photon flux density in the IRGA cuvette was fixed at 1000 µmol m-2 s-1 with an artificial light source. The evaluated parameters were: photosynthesis (A), stomatal conductance (gs), transpiration (E), internal CO2 concentration (Ci) and instantaneous carboxylation efficiency (A/Ci).The results were submitted to variance analysis at 5% and 1% probability by t-test with means and compared by Duncan Test at5% probability.
Biomass production of plants treated with IBA and Stimulate® was approximately 25% higher than the Control treated plants (Table 2). This may have been a consequence of the root system growth, which was 83% (IBA) and 80% (Stimulate®) higher compared to the Control treated ones. This larger plants’ root system was also made evident on the biomass allocation of the roots, about 43% (IBA) and 40% (Stimulate®) higher than the Control treated plants (Table 2). The biomass allocation of the leaves was higher for B+Zn, Tryptophan, Ubyfol® and Control treatments and lower in IBA and Stimulate® treatments (Table 2). Meanwhile, leaf area (LA) was 16% higher in plants treated with Stimulate® compared to the ones receiving Control treatment (Table 2). Plants receiving IBA and Stimulate® treatment had the lowest leaf area ratio (Table 2).
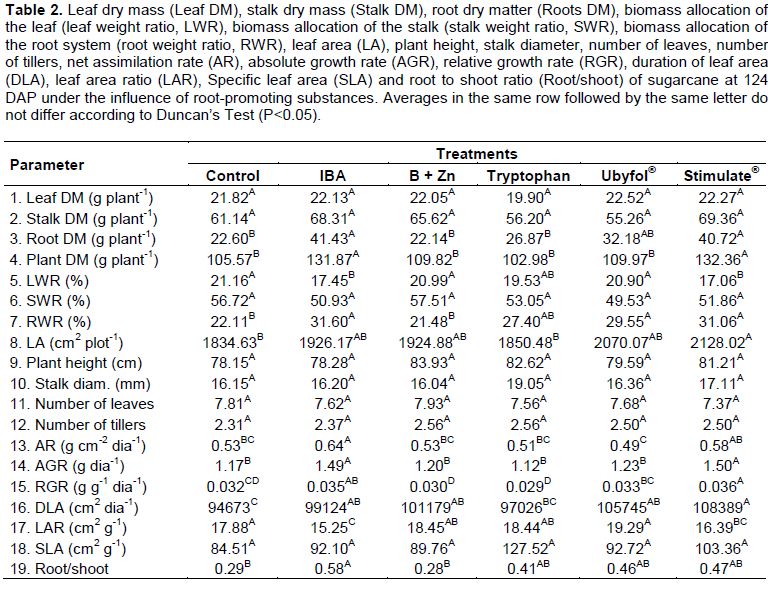
Net assimilation rate was higher for plants treated with IBA (21%) and Stimulate® (9%) and lower for the ones under Ubyfol® treatment (-8%) compared to the Control treated ones (Table 2). Plants treated with IBA and Stimulate® showed relative growth rate about 27% higher than the Control treated ones (Table 2). Plants treated with B+Zn and Tryptophan had the lowest values for relative growth rate, about -6.25 and -9.37%, respectively, compared to Control treated plants.
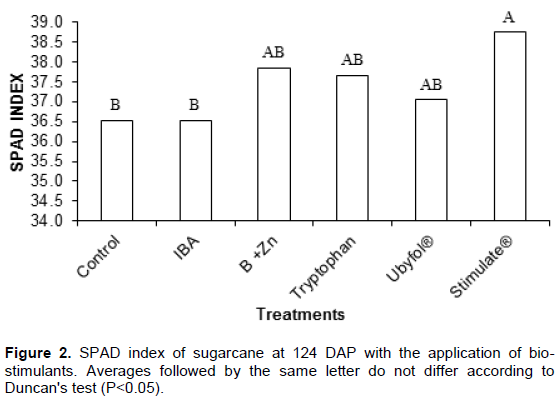
Tryptophan application was the only one not to show an increase on the sugarcane leaf area duration compared to the Control treated ones. The dry mass ratio of root/shoot was significantly higher in plants treated with IBA, Tryptophan, Ubyfol® and Stimulate® compared to Control and B+Zn treatments. The leaf and stalk dry mass, stalk percentage, plant height, stalk diameter, number of leaves, number of tillers and specific leaf area (Table 2) did not differ between treatments. The Stimulate® application increased the sugarcane SPAD index by 6% compared to the Control treatement (Figure 2). The B+Zn, Tryptopnan and Ubyfol® application appear to stimulate the chlorophyll production, however, they have not differed significantly from Control treated ones.
Drought stress caused a Fv/Fm reduction on plants receiving B+Zn, Ubyfol® and Control treatment compared to the hydrated plants (Figure 3A). The plants treated with IAB and Stimulate® had less dynamic photoinhibition, both irrigated and under drought stress plants (Figure 3B). On well hydrated plants, all treatments improved the ΦPSII, and had strong reduction in ΦPSII when under drought condition; however, this reduction was less on Ubyfol® and Stimulate® treatments (Figure 3C).
The Ubyfol® and Stimulate® application increased the stomatal conductance in hydrated plants (Figure 4A). This reflected on the transpiration and photosynthesis of these plants (Figure 4B-C). On drought stressed plants, only Stimulate® treated plants maintained higher stomatal conductance, photosynthesis and transpiration compared to untreated plants.
Plants treated with IBA and Stimulate® had about 43% and 40.5% higher biomass allocation of their roots compared to Control treated plants, respectively (Table 2). Auxin induces the formation of root hairs, as well as lateral root formation (Péret et al., 2011). This increases the root system and probably the water and nutrient adsorption, favoring the absolute and relative growth rate, which was also observed by Verri et al. (1983) on IBA treated sugarcane, and by Dantas et al. (2012) on Stimulate® treated tamarind. Thus, when an auxin biosynthesis inhibitor was applied to tomato seedlings, the root relative growth rate was inhibited (Higashide et al., 2014).
All products used in this work, with the exception of tryptophan, have increased the sugarcane leaf area duration, conveying the time that the photosynthetic leaf area is effectively active, that is, the leaf area magnitude and persistence (Coombs et al., 2014; Costa et al., 2000), potentially enabling greater sugarcane productivity. Similarly, in Sugar beet, the yield was directly affected by the leaf area index and leaf area duration (Cerkal et al., 2007). According to Delgado (1995), the yield potential of a given rice cultivar may be related to the leaf area duration. It was also a strong determinant of biomass yield across genotypes of poplar (Verlindenet al., 2015). The increase in leaf area ratio is related to the improvement of the plant nutritional status, as a result of increased allocation of assimilates for leaf development, resulting in higher leaf weight values and/or leaf area growth (Porter, 1989). Moreover, as the plant grows, there may be a reduction in leaf area ratio due to an interference increase of upper leaves on the lower leaves by self-shading (Benincasa, 1988). This should have been the case in this work (Table 2), since the plants with greater LA showed less leaf area ratio.
The biomass allocation of the leaf and stalk, plant height, stalk diameter, number of leaves, number of tillers and specific leaf area (Table 2) have not differed between treatments. This may be due to the fact that measure-ments were taken at 124 DAP, when the sugarcane had not yet reached its peak growth and carbon accumulation, and it could still be under the influence of plant cuttings reserve used for propagation (Santos et al., 2009). Meanwhile, the following parameters: leaf area ratio, root to shoot ratio (root/shoot), leaf area, root mass, plant mass and biomass allocation of the root system (root %) were good variables to detect changes in the initial growth of sugarcane.
According to Davies (2004), auxins and cytokinins may delay leaf senescence. This may be the reason why treated plants with Stimulate® had higher SPAD readings, since its formulation contains cytokinin and gibberellin.
The PSII maximum quantum yield (Fv/Fm) at predawn indicates chronic photoinhibition when presenting values below 0.7 (Dias and Marenco, 2007). In our experiment, even on plants subjected to stress, Fv/Fm below 0.7 was not observed at predawn, showing that the drought stress was moderate and allowing overnight recovery of the photosynthetic apparatus. According to Bolhar-Nordenkampf et al. (1989), Fv/Fm between 0.75 and 0.85 are characteristic of plants under optimal growth conditions.
A decrease in Fv/Fm throughout the day is an accurate indicator of photoinhibitory damage, when the plants are subject to environmental stresses, including cold and dry (Björkman and Powles, 1984). The plants treated with IBA, Ubyfol® and Stimulate® presented minor damage to the photosynthetic apparatus, that is, less energy loss by photoinhibition along the day, and consequently may have greater conversion of energy into biomass. Meanwhile, the other plants showed a decrease in Fv/Fm, similar to those found by Silva et al. (2007) and Molinari et al. (2007) in sugarcane under drought stress and by Heckathornet al. (1997) in a greenhouse experiment with C4 prairie grasses under drought stress conditions.
The application of biostimulants reduced the photo-inhibition throughout the day and increased the photosystem II yield (ΦPSII) compared to the Control treated plants when the water conditions where adequate. In plants under stress, the ΦPSII reduction was less intense in plants under Ubyfol® and Stimulate® treatments (Figure 3C). Plants under these treatments also showed high root/shoot ratio, allowing a better use of soil moisture. Silva (2010) found ΦPSII values of 0.65 in sugarcane plants subjected to moderate drought stress, and 0.62 when subjected to severe drought stress. In this work, lower values were observed when subjected to drought stress conditions.
The application of Ubyfol® and Stimulate® biostimulants improved the photochemical efficiency and significantly increased the leaves gas exchange (Figure 4), indicating a more intense photosynthetic activity. However, under drought stress conditions, the stomatal closure to minimize water loss through transpiration can trigger the reduction of CO2 diffusion to the substomatal cavity, resulting in smaller photosynthesis activity (Prado et al., 2001). In our work, even in these conditions, plants treated with Stimulate® showed the highest stomatal conductance, transpiration and photosynthesis rates amongst treatments.
The highest stomatal conductance and transpiration rates on Stimulate® treatment under water deficit conditions verified in this experiment, possibly occurred due to these plants greater roots dry mass ratio compared to the other ones, allowing an increase in water and nutrients absorption from the soil. According to Chaves (1991), the maintenance of stomatal opening in drought stress conditions is due to the ability of some plants to extract water from the soil fast enough to compensate for the losses in carbon assimilation. This adaptation can be achieved by plants with deep root systems. It was also found that the Ubyfol® and Stimulate® treatments had the highest rates of photosynthesis; however the Stimulate® treatment presented the highest carbon accumulations with the highest total plant dry mass.a
The application of IBA and Stimulate® treatments enables higher growth rates and biomass accumulation in the initial phase of the sugarcane vegetative growth. When compared to Control treated plants, the application of Ubyfol® and Stimulate® treatments enables greater gas exchange in the absence of drought stress and increases the photosystem II yield even when plants are under drought stress. In plants under moderate stress, Stimulate® treatment application allows higher stomatal conductance, transpiration and photosynthesis rates.
The authors have not declared any conflict of interest.
REFERENCES
|
Albrecht LP, Braccini AL (2009). Aplicação de biorregulador na produtividade do algodoeiro e qualidade de fibra. Scientia Agrari. 10(3):191-198.
Crossref
|
|
|
|
Albrecht LP, Braccini AL, Scapim CA, Ávila MR, Albrecht AJP, Ricci TT (2011). Manejo de biorregulador nos componentes de produção e desempenho das plantas de soja. Biosci. J. 27(6):865-876.
|
|
|
|
|
Artlip T, Wisniewski M (2002). Induction of proteins in response to biotic and abiotic stresses. In: M. Pessarakli (ed) handbook of Plant Crop Physiol. pp. 657-680.
|
|
|
|
|
Ayele N, Dengia A, Getaneh A, Mengistu L, Dilnesaw Z (2014). Effects of bioactivators on yield and yield components of sugarcane at wonji-shoa sugar estate. Int.J.Advanced Res.Biol. Sci. 1:138-143.
|
|
|
|
|
Benincasa MMP (1988). Análise de crescimento de plantas: noções básicas. Botucatu: FUNEP.
|
|
|
|
|
Bertolin DC, Sá ME, Arf O, Junior EF, Colombo AS, Carvalho FLBM (2010). Aumento da produtividade de soja com a aplicação de bioestimulantes. Bragantia 69(2):339-347.
Crossref
|
|
|
|
|
Björkman O, Powles SB (1984). Inhibition of photosynthetic reactions under water stress: interaction with light level. Planta 161:490-504.
Crossref
|
|
|
|
|
Bolhar-nordenkampf HR, Long SP, Baker NR, Oquist G, Shreiber U, Lechner EG (1989). Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Functional Ecol. 3:497-514.
Crossref
|
|
|
|
|
Cairo P, Oliveira L, Mesquita A (2008). Análise de crescimento de plantas. Vitória da Conquista: Edições UESB.
|
|
|
|
|
Cakmak I, Marschner H, Bangerth F (1989). Effect of zinc nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other photo hormones in bean (phasseolus vulgaris). J. Exp. Bot. 40:405-412.
Crossref
|
|
|
|
|
Calvo P, Nelson L, Kloepper JW (2014). Agricultural uses of plant biostimulants. Plant and Soil 383:3-41.
Crossref
|
|
|
|
|
Castro PR (2006). Agroquímicos de controle hormonal na agricultura tropical. Boletim, 32, Série Produtor Rural, uso/esalq/dibd, Piracicaba.
|
|
|
|
|
Cerkal R, Dvorak J, Verjrazka K, Kamler J (2007). The effect of leaf area reduction on the yield and quality of sugar beet (beta vulgaris l. var. altissima döll). Acta Univ. Agric. Silvic. Mendel. Brun. 55(5):37-44.
Crossref
|
|
|
|
|
Chaves MM (1991) Effects of water deficits on carbon assimilation. J.Exp. Bot. 42:1-16.
Crossref
|
|
|
|
|
Choi EY, Kolesik P, Mcneill A, Collins H, Zhang Q, Huynh BL, Graham R, Stangoulis J (2007). The mechanism of boron tolerance for maintenance of root growth in barley (Hordeum vulgare L.). Plant, Cell Environ. 30(8):984-993.
Crossref
|
|
|
|
|
Coombs J, Hall DO, Long SP, Scurlock JMO (eds.) (2014). Techniques in bioproductivity and photosynthesis: pergamon international library of science, technology, engineering and social studies. Elsevier.
|
|
|
|
|
Costa EDC, Santos AD, Zimmermann FJP (2000). Crescimento da cultura principal e da soca de genótipos de arroz irrigado por inundação. Pesquisa Agropecuária Bras., Bras. 35:1949-1958.
|
|
|
|
|
Dantas ACVL, Queiroz JMO, Vieira EL, Almeida VO (2012). Effect of gibberellic acid and the biostimulant stimulate® on the initial growth of tamarind. Rev. Bras. Frutic. 34(1):8-14.
Crossref
|
|
|
|
|
Davies PJ (ed) (2004). Plant hormones: biosynthesis, signal transduction, action!. Springer Science & Business Media.
|
|
|
|
|
Delgado AD (1995). Analisis de crecimiento de la biomasa y el área foliar en la variedad de arroz: Oryzica Yucu-9. Arroz (Colombia) 399:41-48.
|
|
|
|
|
Dias DP, Marenco RA (2007). Fotossíntese e fotoinibição em mogno e acariquara em função da luminosidade e temperatura foliar. Pesquisa Agropecuária Brasileira 42:305-311.
Crossref
|
|
|
|
|
Fachinello JC, Kersten E (1996). Fitorreguladores in: Nachtigal JC, Fachinello JC. Fruticultura-fundamentos e práticas. Pelotas: editora e grafica UFPEL.
|
|
|
|
|
Ferreira júnior RA, Souza JL, Lyra GB, Teodoro I, Santos MA, Porfirio ACS (2012). Crescimento e fotossíntese de cana-de-açúcar em função de variáveis biométricas e meteorológicas. Res. Bras.Eng. Agríc. Ambient. 16(11):1229-1236.
|
|
|
|
|
Garcia RA, Gazola E, Merlin A, Bôas RLV, Crusciol CAC (2009). Crescimento aéreo e radicular de arroz de terras altas em função da adubação fosfatada e bioestimulante/shoot and root growth of upland rice as affected by phosphorus fertilization and biostimulant. Bioscience J. 25: 65-72.
|
|
|
|
|
Goldbach HE, Yu Q, Wingender R, Schulz M, Wimmer M, Findeklee P, Baluska F (2001). Rapid response reactions of roots to boron deprivation. J.Plant Nutrition Soil Sci.164:173-181.
Crossref
|
|
|
|
|
Haggquist ML, Strid I, Widell KO, Lijenberg C (1998). Identification of tryptophan in leachate oat hulls (avena sativa) as mediator of root growth regulation. Physiol. Plant. 72:423-427.
Crossref
|
|
|
|
|
Heckathorn SA, Delucia EH, Zielinski RE (1997). The contribution of droughtâ€related decreases in foliar nitrogen concentration to decreases in photosynthetic capacity during and after drought in prairie grasses. Physiol.Plant. 101:173-182.
Crossref
|
|
|
|
|
Higashide T, Narukawa M, Shimada Y, Soeno K (2014). Suppression of elongation and growth of tomato seedlings by auxin biosynthesis inhibitors and modeling of the growth and environmental response. Sci. Reports 4:4556.
Crossref
|
|
|
|
|
Kramer PJ (1983). Water relations of plants. New York: academic press.
|
|
|
|
|
Liu D, Jiang W, Zhang L, Li L (2000). Effects of boron ions on root growth and cell division of broadbean (Vicia faba L.). Israel J. Plant Sci. 48(1):47-51.
Crossref
|
|
|
|
|
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence—a practical guide. J. Experimental Bot. 51:659-668.
Crossref
|
|
|
|
|
Molinari HBC, Marur CJ, Daros E, Campos MKF, Carvalho JFRP, Filho JCB, Pereira LFP, Vieira LGE (2007). Evaluation of the stressâ€inducible production of proline in transgenic sugarcane (Saccharum spp.): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol. Plant 130(2):218-229.
Crossref
|
|
|
|
|
Péret B, Clément M, Nussaume L, Desnos T (2011). Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci. 16(8):442-450.
Crossref
|
|
|
|
|
Porter NG (1989). Composition and yield of commercial essential oils from parsley. 1: herb oil and crop development. Flavour Fragrance J. 4:207-219.
Crossref
|
|
|
|
|
Prado CHBA, Passos EEM, de Moraes JAPV (2001). Photosynthesis and water relations of six tall genotypes of cocos nucifera in wet and dry seasons. S. Afr. J. Bot. 67: 169-176.
Crossref
|
|
|
|
|
Santos VM, Melo AV, Siebeneichler SC, Cardoso DP, Benício LPF, Varanda MAF (2013). Physiological indices of seedlings of maize (zea mays l.) under the action of biostimulants. J. Biotechnol. 4(3):232-239.
|
|
|
|
|
Santos VR, Moura filho G, Albuquerque AW, Costa PV, Santos CG, Santos ACI (2009). Crescimento e produtividade agrícola de cana-de-açúcar em diferentes fontes de fósforo. Res. Bras.Eng. Agríc.Ambient. 13(4):389-396.
|
|
|
|
|
Schreiber U, Hormann H, Neubauer C, Klughammer C (1995). Assessment of photosystem ii photochemical quantum yield by chlorophyll fluorescence quenching analysis. Func. Plant Biol. 22(2):209-220.
|
|
|
|
|
Serciloto CM (2002) Bioativadores de plantas. Revista Cultivar HF 13:20-21.
|
|
|
|
|
Silva MA (2010). Biorreguladores: nova tecnologia para maior produtividade e longevidade do canavial. Pesquisa e Tecnologia 7:1-10.
|
|
|
|
|
Silva MDA, Cato SC, Costa AGF (2010). Productivity and technological quality of sugarcane ratoon subject to the application of plant growth regulator and liquid fertilizers. Ciência Rural 40:774-780.
Crossref
|
|
|
|
|
Silva MDA, Jifon JL, Silva JAG, Sharma V (2007). Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian J. Plant Physiol. 19(3):193-201.
Crossref
|
|
|
|
|
Sinclair TR, Gilbert RA, Perdomo RE, Brilhar JM, Powell L, Montes G (2004). Sugarcane leaf area development under field conditions in Florida, USA. Field Crops Res. 88:171-178.
Crossref
|
|
|
|
|
Verlinden MS, Broeckx LS, Ceulemans R (2015). First vs. second rotation of a poplar short rotation coppice: above-ground biomass productivity and shoot dynamics. Biomass Bioenergy 73:174-185.
Crossref
|
|
|
|
|
Verri AR, Pitelli RA, Casagrande AAA, Castro PRC (1983). Reguladores vegetais no enraizamento e desenvolvimento de gemas de cana-de-açúcar tratadas termicamente. Anais da Escola Superior de Agricultura Luiz de Queiroz 40:381-394.
|
|