The understanding of the morphology of fruits, seeds and seedlings is very important on the identification and the preservation of species. The work describes and illustrates morphological characteristics of fruits, seeds, germination, and seedlings of Acasia farnesiana. The fruits were collected manually from parent trees selected at random, in the municipality of Sousa State of Paraíba. After collection, they were packed in polyethylene bags and taken to the Seed Analysis Laboratory, Universidade Federal da Paraiba, Areia-PB. Subsequently, evaluations were made of the morphological and morphometric characteristics of fruit, seed germination stages, and seedling. The fruit is of type nucule vegetable, plain, dry, polysemy, glabrous, linear-format wavy and late dehiscence. The seeds are stenospermics, format obovoid, apex rounded, and base slightly rounded. The germination is epigeal - phanerocotyledonal, starting on the third day and may be terminated on the seventh day after sowing. It was possible to describe and illustrate in detail the morphology of fruit, seeds, germination stages, and seedling of the species, considering the characteristics of easy recognition, standardization, rapid achievement, and high probability of establishment in the field.
Acacia farnesiana L. Willd. is trees or shrub, which belongs to Fabacea family and Acacia genus. It has large distribution in tropical and sub-tropical areas all over the world due to its high capacity of adaptation to diverse weather conditions, as well as geographic and altitude conditions, making the adaptation of this species easy in different environments (Erkovan et al., 2013). This aspect has facilitated its introduction in different regions. In addition, several species of this genus are used as ornamental lumberyard, in tanneries due to the presence of tannins, for extraction of gums. Its flower essences are used in perfumery, fixing dunes, and formation of hedges (Ramli et al., 2011; Kingsley et al., 2014).
For wild plants, one of the biggest problems found by experts is the lack of information related to species identification, once not always identified botanical material is available (Amaro et al., 2006).
In Leguminosae family, subfamily Mimosoideae, vegetative and floral characters, in which the systematic study of Angiosperm is fundamentally based, are not always sufficient for characterization of some taxonomy groups, reason why the fruits and seeds have been used as a decisive character. Without these, recognizing certain genus become difficult (Camacho et al., 2012). According to the author, morphological descriptions of such structures are usually quite large at subfamily level or they are found in brief generic diagnoses. The morphological identification of seedlings also allows to characterize families, genera, and even species, having been applied in studies of forest inventories in regions of temperate and tropical climates (Anez et al., 2005).
In the environment, there is morphological diversity of fruit that in the elapse of their evolution have passed through a series of adaptation and have acquired different forms (Cosmo et al., 2009; Paoli and Bianconi, 2008). Thus, the description of the fruit within an ecological context is a new way to understand the biology of reproduction (Anez et al., 2005). Biometrics of fruits and seeds, as well as the knowledge of morphology and seedling development is critical to support studies of germination and seedling production for plant restoration (Leonhardt et al., 2008).
About the development of seedlings, Cosmo et al. (2009) reported that this stadium deserves proper attention, because it can be used to elucidate morphological and anatomical aspects, other than promoting species’ recognition of certain regions in ecological studies. In this context, Silva (2012) stated that information about the development stage can be used with great reliability for such purposes, helping the immediate identification of the species.
Despite the importance of morphological studies of seedlings, there are a few studies of that in Brazil, especially when it comes to native forest species, like this study was conducted aiming to characterize morphologically fruits, seeds, germination, and seedlings of A. farnesiana.
Mature fruits of A. farnesiana were harvested by hand from matrix plants located in Sousa State of Paraíba and were put in plastic bags and carried to the Laboratory of Seed Analyses, Department of Fitotecnia and Environmental Science, in the Center of Agricultural Science of the Federal University of Paraiba, in Areia–PB, Brazil. There, fruits were handly opened to remove the seeds.
Morphological characterization of fruits and seeds
For biometric determinations, hundred fruits and seeds were selected randomly for individual measurement of length, width, and thickness, using a digital caliper with a precision of 0.001 mm. The length was measured from the base to the apex, excluding the stalk and the width and thickness measurements were measured in the midline of the fruits and seeds (CHEIB, 2009); after measurement of fruits and seeds, they were individually weighed on an analytical balance accurate to 0.001 g.
The quantitative characteristic data were submitted to descriptive analysis, calculated with Excel application, arithmetic mean, standard deviation, coefficient of variation, range of variation, and relative frequency of fruit and seeds.
The considered aspects to describe fruits were weight, type, color, size, texture and consistency of the pericarp and seed number per fruit; in seeds, the external morphological characteristics were weight, type, color, size, texture, and consistency of integuments, form, edge, position of the hilum, micropyle, and raphe, while the intern characteristics observed were embryo (cotyledon, hypocotyl-radicle axis, plumule) and the presence of endosperm.
To make the intern morphologic study of seeds easier, they were manually scarified with sandpaper number 80 in the side opposite the hilum region and then immersed in water at room temperature for a period of 24 h.
In addition to the analyzes described earlier, the weight of a thousand seeds was determined, using eight replicates of 100 seeds each, following the methodology described in the Rules for Seed Analysis (Brasil, 2009a).
Morphological characterization of germination and seedling
The methods and terms used to describe morphologic aspects of fruits, seeds, germination, and seedlings of A. farnesiana are according to Barroso et al. (1999), Brasil (2009b) and Silva et al. (2012), and were drawn by hand.
Morphologic characterization of fruits
The fruit of A. farnesiana is legume nucoide, simple, dry, polysemy (presenting 10 to 27 seeds), glabrous, straight-wavy shape, and late dehiscence (Table 1). This species of fruit has a pleasant odor, depressions on the sides and on one suture line in the ventral and dorsal portion from the stem to the apex. The base is acuminate, stipellate with persistent stems and ligneous consistency (Figure 1A). The epicarp is dry, opaque, with slightly striated surface, glabrous and dark brown; the mesocarp is dry, with light brown color and consistency of cork-fibrous, which is divided by false septa where the seeds are (Figure 1B); it is possible see to one yellowish sticky substance between the epicarp and mesocarp.
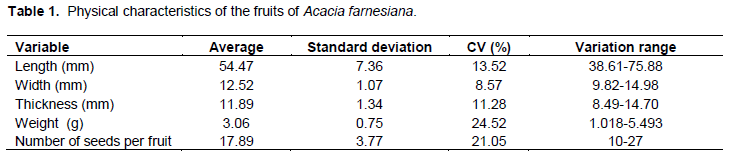
The legume fruit has origin from the supper ovarian, which is unicarpellate, dehiscent at the junction point of the edges of the carpel and the dorsal region of the midrib, forming two valves. Those are characteristic only of the Leguminosae family, who is open along two sutures (often of equal length, nonligneous) causing the separation of the valves which keep them together in the base (Barroso et al., 1999).
Studying fruits of Erythrina velutina Willd., Silva et al. (2008) observed similar results regarding fruit type and coloration. Likewise, Abud et al. (2009) assessed the fruit morphology of Mucuna aterrima (Piper and Tracy) and got the same information related to the fruit type, epicarp, and seed numbers per fruit.
Similar results were found by Nogueira et al. (2010) studying the fruit morphology of pau-violeta (Dalbergia cearensis Ducke) looking for the irregular form of size and length, which ranged from 30.61 to 47.57 mm. In addition, the width was between 7.83 and 10.88 mm, and the thickness around 2.94 and 4.95 mm.
Caatinga species in conditions of water stress caused by drought have functional changes, such as anatomical and morphological changes, including deep root growth, decrease in leaf size, stem growth and leaf loss (Trovão et al., 2007). Therefore, it is believed that changes in size of fruits and seeds are not only related to genetic resources, but also to the conditions determined by the environment (Figure 2).
Morphological characterization of seeds
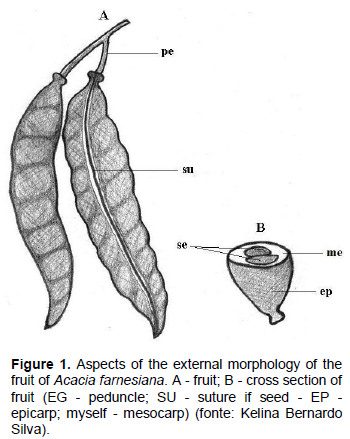
The seeds of A. farnesiana are stenospermics of obovoid shape, apex rounded, and slightly rounded base (Figure 3). The obovoid shape was described by Barroso et al. (1999) referring to seeds of other species of Acacia. The testa is smooth, dark brown color, hard consistency when dried, and leathery when hydrated. This agrees with Sousa (2010) when he reported that the testa of the leguminous seeds is usually brown or black.
The seed has on both sides apical-basal pleurograma, which represents about 85% of its length; the hilum is small, circular format, located at the base of the seed (Figure 3A); the micropyle has spot-on format and is located just below the hilum, being barely noticeable to the naked eye and the raphe is absent. The storage tissue is cotyledonary, whitish color, firm consistency, crass flats, obovate form, opposite and equals. On the base of the cotyledons, there is a small crack, with approximately 1 mm in length (Figure 3B). The axis of the hypocotyl and radicle is short, straight, and does not go up until the cotyledons (Figure 3C). The seedling is not visible and is devoid of mature seed endosperm, with all the material stored in the cotyledons, so that they occupy the entire length of the seed.
Variations in color, shape, and seed size were described to some species from the caatinga (Araújo et al., 2007); however, according to Araujo et al. (2007) there is little information about morphological variations of the seeds from the caatinga, making difficult a broader discussion about the importance of these changes for successful seed germination and seedling establishment.
The average length is 6.97 mm (range 4.69 to 8.20 mm); average width of 5.43 mm (range 3.95 to 7.11 mm); average thickness of 3.49 mm (range 2.90 to 4.50 mm); average weight of 0.11 g (ranging from 0.062 to 0.152 g) and thousand seed weight of 105.6 g (Table 2). These results are similar to those obtained by Battilani et al. (2011) who studied the morphology of Guibourtia hymenifolia (Moric.) J. Leonard.
The results of frequency analysis of the seeds (Figure 4A to D) indicated predominance of 66% with lengths ranging from 6.45 to 7.33 mm, 49% width ranging from 4.74 to 5.53 mm, 57% thickness ranging from 3.3 to 3.7 mm and 54% weight ranging from 0.108 to 0.131 mm.
In most of the habitats, seed size varies from ten orders of magnitude, although within the same species this size represents less than half of this variation (Brito et al., 2014). Species with large seeds have greater persistence and establishment of a broad range of environmental conditions, while species with small seeds are more perturbation dependent. Large seeds increase seedling survival. However, in order to understand that in relationship it is necessary to have knowledge of how the reserves of seed are used during germination and early seedling establishment (Nogueira et al., 2010).
The weight of the seed has a strong influence on the plant establishment, since the heavier seeds often originate seedlings with larger initial length and better survival in a low light environment (Parker et al., 2006). On the other hand, to Nogueira et al. (2010) small seeds have greater ease in obtaining water for germination than large seeds, due to the higher surface/volume ratio. Thus, because of its size, seeds of A. farnesiana have advantages for semi-arid conditions of the Brazilian Northeast, since water availability is restricted to a short rainy season.
Germination characterization
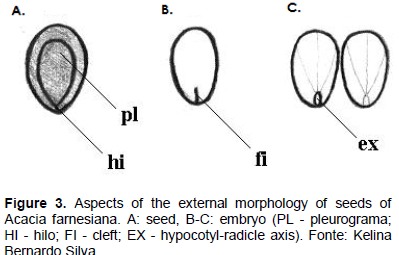
The swelling of the seed can be observed 24 h after seeding, and on the third day the root protrusion in the micropyle region is observed, which is thin, conical, glabrous, and white in color, having an expressive development (Figure 5A to B). Subsequently, with the loosening of the integument, arises the paracotyledons with a yellowish green color, obovate shape, opposite, glabrous, crass, with a visible crack in the base, which are the first photosynthetic organs of the seedling (Figure 5C).
Initially, the hypocotyl is short, straight, bright green in color, glossy and, as it grows, there is a differentiation of the root due to the increased width, shown by the larger diameter of the hypocotyl, where the cervix is located (Figure 5C). From the sixth day after sowing, the paracotyledons are semi-open, appearing among them protophilus of the first order (Figure 5D); epicotyl is very small and barely noticeable; still at this stage, the first secondary roots emerge just below the cervix (Figure 5D).
On the seventh day after sowing, the protophilus of the first order are fully expanded and appositive, and may terminate the germination phase; the seedling is pivoting brownish, with average length of 105 mm root. The hypocotyl is bright green, cylindrical, smooth, glabrous, and straight, with average length of 55 mm; during this stage the paracotyledons have dark a green coloration, are crass, dull and persistent, with cordate base, obtuse apex, and hole board and obovate shape. The epicotyl is short, straight, cylindrical, thin, bright green, and glabrous. At the apex is the apical, while the first couple of protophilus are opposites, both being composed of a pinna. The pinnae are par pinnate, with 9 pairs of green leaflets-dark and membranous consistency; each leaflet with a base and obtuse apex, sepsis without apparent nervous and entire margin (Figure 5E).
The germination of A. farnesiana seeds is considered by Silva et al. (2008) as epigeal and phanerocotylar, and the most common form of germination for most legumes.
Morphological characterization of seedlings
From the 15th day after sowing, change of phyllotaxis in seedlings of A. farnesiana is observed, with the presence of the first pair of pinnate, which are opposed and unique, and are followed by pinna position switches, two in number; at the base of the pedicel of the pair of pinnae two prominent stipules (visible and opposite) are formed. The epicotyl is straight, thin, glabrous and reddish, and at this stage, the primary root has tender consistency and secondary roots become abundant; paracotyledons in the seedlings are persistent and measure approximately 150 mm long (Figure 6).