ABSTRACT
The presence of dormant seeds makes it difficult to evaluate physiological quality and requires the use of appropriate methods in order to break seed dormancy. The objective of this study was to evaluate the effectiveness of different methods to break the dormancy of ryegrass seeds stored under environment conditions in different periods. Ryegrass seeds, BRS Ponteio cultivar, produced in two locations were used and evaluated after 60, 90, 120, 150 and 180 days of storage under environment conditions. Seeds were submitted to the following methods to break dormancy: sowing at 20 to 30°C without applying any method to break dormancy (control); pre-cooling (5°C) for 3 days + KNO3 followed by sowing at 15 to 25°C; pre-drying (45°C) for 96 h followed by sowing at 20-30°C; immersing the seeds in sodium hypochlorite (NaClO) at 0.5% for 24 h, followed by drying at 45°C for 6 h and sowing at 15 to 25°C. At 60 days after harvest, the most effective method was immersion of seeds in a NaClO solution followed by drying at 45°C. From 90 days after harvest, all methods were equally effective to break seed dormancy, except pre-drying (45°C) for 96 h, which negatively affected the physiological quality of seeds. There is a difference in the effectiveness of the methods employed to break the dormancy of ryegrass seeds depending on the type of post-harvest storage.
Key words: Lolium multiflorum, post-harvest storage, pre-cooling.
Ryegrass (Lolium multiflorum Lam.) has a widespread occurrence in southern Brazil, especially in Rio Grande do Sul (RS) state, where it is used as forage for livestock and soil cover in agricultural areas during the winter period. This forage species can be considered important for the agricultural context in southern Brazil due to its growing cycle complementarity with natural pastures, high nutritional value, ease of establishment and excellent capacity of natural reseeding (Costa et al., 2013).
However, Lacerda et al. (2010) suggested that approximately 80% of Brazilian pasture areas are degraded. The low germination rate of seeds causes such problem and may be related to a phenomenon called dormancy, i.e., seeds do not germinate even when exposed to favorable environmental conditions. This phenomenon is one of the main strategies used by plant species to increase their survival rates and establish young plants. It thus provides an additional period for its natural dispersion (Taiz and Zeiger, 2013). The dormancy mechanism has peculiarities according to species, making the generalization about its causes complex. It may occur independently or in combination as in most grasses (Previero et al., 1998).
However, the presence of dormancy in forage species seeds is fundamental, allowing them to survive the unfavorable period of the summer and germinate only in the fall, when environmental conditions are suitable for the development of this culture.
As mentioned by Baldi et al. (2012), dormancy often is not a problem for the users of seeds since they tend to break naturally between the sowing and the harvesting of the culture. However, it is a partially problem for seed production, dormancy soon after harvest makes the evaluation of the physiological quality and the implementation of measures related to quality control of production difficult, which requires appropriate methods to break the dormancy of seeds. Furthermore, it is known that, for many forage species, the effectiveness of methods designed to break dormancy varies depending on the age of the seeds (Costa et al., 2011) and that the storage itself is enough to promote dormancy breaking.
In this context, studies on breaking the dormancy of seeds have been conducted. However, studies are scarce when it comes to forage species. Popinigis (1985) indicated several methods to break the dormancy of grass seeds. Among the main methods, there are rupture of caryopsis, treatment with potassium nitrate (KNO3), exposure to light, use of alternating temperatures, pre-cooling and treatment with hormones (gibberellin, cytokinin and ethylene).
The objective of this study was to evaluate the effectiveness of different methods to break the dormancy of ryegrass seeds stored under environment conditions in different periods.
The experiments were conducted at the Temperate Embrapa Seed Analysis Laboratory in Capão do Leão/RS and at the teaching laboratory of seed analysis "Flavio Rocha" of the Faculty of Agronomy "Eliseu Maciel" of the Federal University of Pelotas between January and June 2014. Annual ryegrass seeds (Lolium multiflorum Lam.), BRS Ponteio cultivar, produced in Pedras Altas/RS and Capão do Leão/RS, were used. Seeds were collected, processed and stored under non-controlled environment conditions and evaluated at 60, 90, 120, 150 and 180 days after harvest. The following methods were used to break the dormancy of seeds:
Method 1: Sowing at 20 to 30°C without applying any method intended to break seed dormancy: four replications using 100 seeds were sown on two sheets of blotting paper moistened with distilled water in an amount equal to 2.5 times the dry mass and kept under alternating temperatures of 20 to 30°C. The seeds were evaluated 14 days after the test installation and the percentage of germination was recorded.
Method 2: Pre-cooling (5°C) for 3 days + KNO3 followed by sowing at 15-25°C: four replications of 100 seeds were sown on two sheets of blotting paper moistened with a potassium nitrate (KNO3) solution at 0.2% in an amount equivalent to 2.5 times the dry mass and stored at 5°C for three days. After this period, seeds were transferred to a Biochemical Oxygen Demand (BOD) chamber with alternating temperatures of 15-25°C and a photoperiod of 8 hours. The percentage of germination was evaluated 14 days after the installation of the germination test.
Method 3: Pre-drying (45°C) for 96 h followed by sowing at 20-30°C: the methodology was similar to that described for the Method 1, except that the seeds were subjected to a pre-drying at 45°C for 96 h prior to the installation of the germination test.
Method 4: Immersion of seeds in sodium hypochlorite (NaClO) at 0.5% for 24 hours, followed by drying at 45°C for 6 h and sowing at 15-25°C. The methodology was similar to that described for Method 1, with the difference that seeds were soaked in sodium hypochlorite (NaClO) at 0.5% for 24 h followed by washing in water. Seeds were dried at 45°C for 6 h.
The experiments were arranged completely randomized design with four replications in a 5 (storage period) x 4 (treatment) factorial design. Treatments consisted of a combination of five storage periods after harvest and four methods for breaking seed dormancy. The data were transformed into arcsin (x/100)1/2 and subjected to analysis of variance. The averages were compared by Tukey test (p<0.05).
It was observed that, at 60 days after seed harvest, the most efficient method for breaking seed dormancy was immersion in sodium hypochlorite (NaClO) at 0.5% for 24 h, followed by drying (45°C) for 6 h and sowing at 15-25°C (Tables 1 and 2). In this case, seeds produced in Pedras Altas/RS that have not undergone any method to break seed dormancy had 49% of germination, which increased to 69% after the procedure to break seed dormancy (Table 1). For seeds produced in Capão do Leão/RS, this method increased the germination of seeds from 87% to 99% (Table 2). Using rice seeds, this method was also the most efficient after pre-drying at 45°C for 96 h to break seed dormancy of the cultivar IRGA 425 and the cultivar SCS 114 Andosan (Baldi et al., 2012). According to these authors, the advantage of this method is the possibility of using mechanical counters for the sowing of seeds after the procedure because seeds are dried. These results are in agreement with those obtained by Vieira et al. (1994), who studied the effects on rice seeds by soaking them into a sodium hypochlorite solution for different periods (24, 36 and 48 h). They concluded that this method was not effective in breaking seed dormancy.
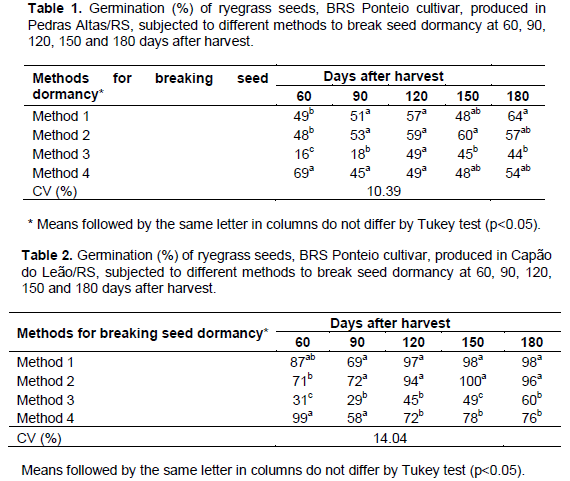
Unlike the results found for rice seeds, the pre-drying at 45°C for 96 h (Method 3) was detrimental to the physiological quality of seeds because it reduced the germination throughout the entire experiment. It was not recommended as a method to break the dormancy of ryegrass seeds. Studies conducted with forage species reveal that the optimum temperature for germination is specific for each cultivar and that seeds do not germinate at temperatures above 45°C (Seepaul et al., 2011).
Importantly, many methods employed to break seed dormancy immediately after harvest are not completely effective for several forage seeds, resulting in a high percentage of dormant seeds after the germination test, as noted by Eichelberger et al. (2001) for ryegrass seeds and confirmed by the results obtained in this study. Thus, after 90 days of storage, although all methods were equally effective to break seed dormancy (except for pre-drying at 45°C for 96 h, which damaged the physiological quality of seeds in all storage periods), it was observed that the expression of the maximum germination potential of the seeds was not achieved. It remained even below the values ​​observed at 60 days after harvest (Tables 1 and 2).
The storage of ryegrass seeds produced in Capão do Leão/RS for 120 days seems to have been enough to promote a natural break of dormancy since, after this period, seeds not subjected to any method to break dormancy showed a 97% germination rate (Table 2). This period is very close to that observed for natural dormancy breaking of ryegrass seeds and reported by Costa et al. (2013). Research shows that, in freshly harvested seeds, the water ingress in tissues hinders the absorption of oxygen and that the storage of dry seeds for a certain time promotes the diffusion of oxygen into the interior, determining a reduction in the amount of germination inhibitors and favoring breaking seed dormancy (Duclos et al., 2013).
From 120 days of storage, the pre-drying of the seeds (45°C) for 96 h and the immersion in NaClO solution followed by drying were harmful to the physiological quality of seeds produced in Capão do Leão/RS. It reduced the germination to 45 and 72% respectively after 120 days of storage, to 49 and 78% respectively after 150 days of storage, and to 60 and 76% respectively after 180 days of storage (Table 2). As the physiological maturation process of ryegrass seeds is uneven, it is likely that seeds that were already at an advanced stage at harvest suffered a greater deteriorating stress over the storage period, being affected by the immersion in NaClO (Method 4). Lima et al. (2012) used the immersion of coffee seeds in an aqueous solution of sodium hypochlorite at concentrations of 3, 4, and 5% of active chlorine, resulting in an acceleration of germination.
This behavior has been highlighted by Eichelberger et al. (2001) for ryegrass seeds subjected to pre-cooling. The authors state that, because of dormancy, the germination test conducted soon after harvest may underestimate the quality of seeds even after the adoption of pre-cooling as a method to break seed dormancy. The findings of this study corroborate the data found by Amaro et al. (2012). They concluded that, upon evaluating dormancy breaking methods in basil (Ocimum basilicum L.) and, after seed storage, this same method may cause stress to the seeds, confusing the results of the germination test. These results were observed for the seeds produced in Pedras Altas/RS, whose germination after 120 days of storage was not affected by any method intended to break seed dormancy (Table 1).
After 150 and 180 days of storage, the only method that negatively affected the germination was pre-drying (45°C) for 96 hours (Table 1). It is known that dormancy and seed quality in general are greatly affected by environmental conditions during the process of seed formation. This may explain the different behavior of seeds produced in Pedras Altas/RS and Capão do Leão/RS regarding the effectiveness of the methods used to break seed dormancy and the quality of obtained seeds.
The officially recommended method to break the dormancy of ryegrass seeds (pre-cooling at 5°C for 3 days) was not effective in any of the evaluated periods for seeds produced in the two locations. This resulted in a germination rate statistically similar to the germination of seeds that did not undergo any treatment to break seed dormancy in all situations (Tables 1 and 2).
There is a difference in the effectiveness of the methods employed to break the dormancy of ryegrass seeds depending on the type of post-harvest storage. The immersion in NaClO solution at 0.5% for 24 h followed by drying (45°C) for 6 h was effective in breaking the dormancy of seeds stored for up to 88 days after harvest. The method pre-drying (45°C) for 96 h was detrimental to the physiological quality of seeds. It was not recommended as a method to break seed dormancy.
The authors have not declared any conflict of interests.
REFERENCES
|
Amaro HTR, Assis MO, David AMSS, Silveira JR, Silva Neta IC, MOTA WF (2012). Superação de dormência em sementes de manjericão (Ocimum basilicum L.). Rev. Bras. Plant Med. 14:218-223.
Crossref
|
|
|
|
Baldi ME, Segalin SR, Barzotto F., Andrade FF, Mattioni NM, Mertz LM (2012).Métodos alternativos para superação da dormência em sementes de arroz irrigado. Inform. Abrates 22(2):16-19.
|
|
|
|
|
Costa CJ, Araújo RB, Villas Bôas HDC (2011). Tratamentos para a superação da dormência em sementes de Brachiaria humidicola (Rendle) Schweick. Pesqui. Agrop. Trop. 41(4):519-524.
|
|
|
|
|
Costa CJ, Mittelmann A, Silva MG, Ribeiro PRG, Vaz CF, Franco DF(2013). Superação da dormência em sementes de azevém da cultivar BRS Ponteio. Pelotas: Embrapa Clima Temperado,17 p. (Boletim de Pesquisa & Desenvolvimento 196 p.)
|
|
|
|
|
Duclos DV, Ray DT, Johnsonb DJ, Taylor AG (2013). Investigating seed dormancy in switchgrass (Panicum virgatum L.): understanding the physiology and mechanisms of coat-imposed seed dormancy. Ind. Crops Prod. 45:377-387.
Crossref
|
|
|
|
|
Eichelberger L, Maia MS, Camacho JCB (2001). Períodos de pré-esfriamento na superação da dormência de sementes de azevém-anual (Lolium multiflorum Lam). Rev. Bras. Sem. 23(1):212-218.
Crossref
|
|
|
|
|
Lacerda MJR, Cabral JSR, Sales JF, Freitas KF, Fontes AJ (2010).Quebra da dormência de sementes de Brachiaria brizantha cv. Marandu. Rev. Semina 31(4):823-828.
|
|
|
|
|
Lima JS, Araujo RF, Dias LAS, Dias DCFS, Rena FC (2012). Uso da reidratação e do hipoclorito de sódio para acelerar a emergência de plântulas de cafeeiro. Rev. Bras. Sem. 34:327-333.
Crossref
|
|
|
|
|
Popinigis F(1985). Fisiologia da semente. Brasília, DF: AGIPLAN 289 p.
|
|
|
|
|
Previero C A, Groth D, Razera L F (1998). Dormência de sementes de Brachiaria brizantha (Hochst. Ex A. Rich) Stapf armazenadas com diferentes teores de água em dois tipos de embalagens. Rev. Bras. Sem. 20(2):392-397.
Crossref
|
|
|
|
|
Seepaul R, Macoon B, Reddy KR, Baldwin B (2011). Switchgrass (Panicum virgatum L.) intraspecific variation and thermotolerance classification using in vitro seed germination assay. Am. J. Plant Sci. 2:134-147.
Crossref
|
|
|
|
|
Vieira AR, Vieira MGGC, Carvalho VD, Fraga AC (1994). Efeitos de tratamentos pré-germinativos na superação da dormência de sementes de arroz e na atividade enzimática da peroxidase. Pesqui. Agropecu. Bras. 29(4):535-542.
|
|
|
|
|
Taiz L, Zeiger E (2013). Fisiologia vegetal. 5. ed. Porto Alegre: ArtMed 954 p.
|
|