Vitex trifolia L. was found in several countries such as southern Africa, Madagascar and Mauritius to Afghanistan, India, Sri Lanka, Burma (Myanmar), Indo-China, southern China, Japan, Thailand, throughout the Malesian region, south to northern Australia, east to New Caledonia and Indonesia (Capareda, 1999). V. trifolia L. is one of thousands medical plants that grow in Indonesia. The plant was known as an annual plant which has many beneficial. Until now, information about the marker compound Vitexicarpine in the medical plant V. trifolia L. is still scarce. Some Indonesian traditional therapists believe that the leaves of V. trifolia could be used as a medicine for antiasthma, anti-allergy and anticancer. In India, Murugan and Mohan (2012) used the plant as an antifeeding activity against the insect pest Spodoptera frugiperda, antifungal activity, and antibacterial activities.
The leaves of V. trifolia contain a volatil oil composed of sesquiterpen, terpenoide, ester compound, alcaloid (vitrisin), glycoside flavon (artemetin and 7-desmetil artemetin) and non flavonoid friedelin components, ß-cytosterol, glucoseand hydrocarbon compound which has analgesic and anti allergy (Alam et al., 2002). Vitexicarpinis known as a pharmacologic active component in the leaves of V. trifolia and suitable as a marker compound (Alam et al., 2002; Ikawati et al., 2001). Therefore, V. trifolia L. is a prospective plant to be developed as medical plant in an industry scale. For the purpose, the plant should be grown in a good environment and soil quality (Maheswari, 2002). Information about relationship between a marker compound in the plant and soil characteristics is still so scarce, also still rare information on the relationship between the content of a marker compound in the plant with the soil characteristics where the plants grow. The main objective of this research is to find out relationship of soil quality index and vitexicarpine content in the leaves of V. trifolia.
This research was conducted in 7 locations, at sub district of Tawangmangu and Karangpandan. The two locations were situated in District of Surakarta (Province of Central Java) and the five were situated in district of Kulon Progo (Province of Yogyakarta). Soil types where the V. trifolia grew on it were described. Based on diagnostic horizon characteristics of the both soil profile from Tawangmangu and Karangpandan could be classified as Andisols (according to soil taxonomy USDA). Whereas, soil types collected from the five locations situated in Kulon Progo were two Entisols and three Inceptisols. Soil physical and chemical analyses was done for soil texture (sand, silt and clay fractions were determined by pipette method), porosity (n), bulk density (wax method), pH (electrode pH-meter), organic carbon (Walkey and Black), total nitrogen (Kjeldahl method), available phosphorous (Olsen method), K-available, Ca-available and Mg-available (extracted by NH4OAc, pH 7 and measured by AAS). Soil quality index was calculated based on the soil physico-chemical properties by referred to Karlen et al. (1996). Procedure for Vitexicarpine analysis was done as follow: dried leaves extracted with ethyl acetate, concentrated in vacuo and further analyzed for aglycone vitexicarpine. The residue was dried from ethyl acetate, extracted with methanol 50% in water and concentrated in vacuo. Methanol extract was hydrolyzed in acidic condition (HCl 10%, 80°C, 1 h) and extracted by ethyl acetate. The ethyl acetate fraction was concentrated in vacuo and further analyzed the vitexicarpine as glycoside vitexicarpine. Vitexicarpine content analyzed by thin layer chromatography with stationary phase silica gel F254 (Merck), mobile phase n-hexane:ethyl acetate (2:1 v/v), and 8 cm migration distance. The chromatogram was scanned in TLC Scanner 3 (Camag) at wavelength 346 nm (Alam et al., 2002).
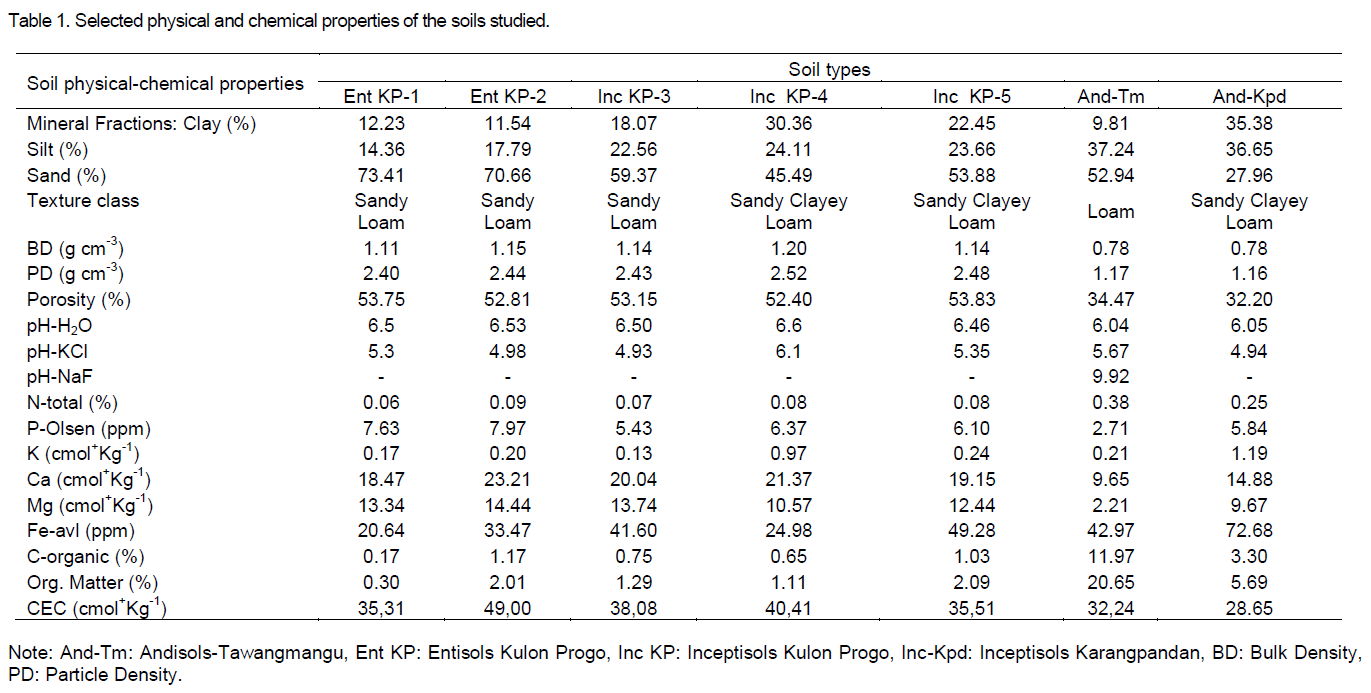
Physical and chemical properties of the soils
Physical and chemical properties of soils under V. trifolia L. plantation are presented in Table 1. In general, the authors observed three orders of soils, namely: Andisols, Incepstisols, and Entisols. The soil physical properties such as texture (proportion of sand, silt and clay), bulk density, particles density and porosity were in a range of sandy clayey loam–loam, 0.78-1.2 gcm-3, 1.17-2.52 gcm-3 and 34.47-53.83%, respectively. The soil reaction could be categorized as slightly acid-neutral (pH-H2O, 6.04-6.6). The concentration of N, P, K and C-organic (except for Andisols in a high rate) in all of the soil types were categorized as a low rate. In case of Ca2+, Mg2+ and Fe content was categorized as medium-high rate (SRC, 2009).
Vitexicarpine content in the leaves of V. trifolia
Analysis of V. trifolia L. leaves indicated that vitexicarpine content in the leaves was a wide variation. It is likely to related to leaves maturation and location where the leaves collected (presented in Figure 1). Aglycone vitexicarpine content is higher in young leaves (0.36-0.5%) than older leaves (0.22-0.33%). As levels of free forms (Aglyconevitexicarpine), levels of bound forms (glycoside vitexicarpine) also showed similar results. Young leaves had a higher concentration (0.32 to 0.53%) than older one (0.24-0.42%). This result is surprising considering the higher vitexicarpine content was found in the leaves with low maturation level.
Proportion between the aglycone vitexicarpine and glycoside vitexicarpine content was not depend on the leaves maturation level (Murti et al., 2009). In general, the content of the glycoside vitexicarpine was higher than the aglycone vitexicarpine. This will lead to the consequences of selecting the optimal solvent for extraction of the V. trifolia L. leaves.
Comparing among the sample locations indicated that the total vitexicarpine content in the leaves was so various (Table 3), in the young and old leaves were observed at a range of 0.73-1.03% and 0.46-0.75%, respectively. In average, the total vitexicarpine concentration in the young leaves was significantly higher than the old one. Therefore, for the purpose of standardizing the leaves extract of V. trifolia would be better if in the process of harvesting young leaves have just taken. Among the 7 locations indicated that there are 3 locations where the vitexicarpine content in the leaves was higher than the other places, namely: Kulon, Progo (only for KP-1 and KP-2) and Tawangmangu. Thus the three locations were fit for use as a raw materials source of the V. trifolia leaves extract. Alam et al. (2002) proposed the chemical structure of the vitexicarpine presented in Figure 2.
Soil quality index (SQI) and Vitexicarpine content
Soil quality is the capacity of a soil to function within ecosystem boundaries to sustain biological productivity, maintain environmental quality, and improve the health of plants and animals (Doran and Parkin, 1994; Karlen et al., 1996). Soil quality index is an index based on the value and weight of each indicator of soil quality. Soil quality indicators were selected from properties that show the capacity of the soil functions that determine the level of soil fertility. Indicators of soil quality are the nature, characteristics or physical processes, chemical, and biological soil that can describe the condition of the soil (Doran and Parkin, 1994; Andrews et al., 2004). Based on the calculation of the soil quality index of each research area are presented in Table 2.
According to Wander et al. (2002) based on scoring functions indicated soil quality rate for a particular land use determined from 0-1. Soil quality index values closer to the value of one the better soil quality. Based on Table 2, it was known that the soil quality index of the seven research sites could be categorized at a range of low to very good rating. Soil samples from Kulon Progo 1 and 2 could be classified as Entisols order and have SQI values of 0.274 and 0.315, respectively and the both value could be categorized as a low soil quality rate. While the soil samples from the Kulon Progo 3, 4 and 5 could be classified as Inceptisols order and have SQI values of 0.452, 0.580 and 0.541 respectively, and all the three value could be categorized as a medium soil quality rate. Soil which has a very good rate in the soil quality is Andisols orders found in Tawangmangu and Karangpandan (0.818 and 0.991, respectively).
Based on Table 3, it is known that the location of study has a soil quality index and vitexicarpine content in the leaves of V. trifolia diverse. The relationship between soil quality index and vitexicarpine concentration in the leaves of V. trifolia was a negative correlation (Figure 3).
This means that the higher the soil quality index is the vitexicarpine concentration in the leaves of V. trifolia relatively is lower. This is due to the plant has a capability for survival through a certain metabolism mechanism in the plant cell if under a high environmental pressure. Soil quality index of Andisols from Karangpandan-Surakarta was 0.991 and vitexicarpine content in the leaves was only 0.6%, but in case of Entisols, the soil quality index was 0.274 and vitexicarpine content was able to reach around 0.82%. Inceptisols quality index was an intermediate between Andisols and Entisols.
In general, it has been known that soil is an important factor controlling the plant growth. Nutrient availability in soil is needed to produce the secondary metabolite compounds in the plant. There are several soil nutrients has an important role in synthesis of vitexicarpine, such as: nitrogen, magnesium and iron. Nitrogen and magnesium has an important role in synthesis of flavonoid compound (Torsell, 1997). Nitrogen also has an important role in synthesis of amino acid and protein in the plant (Epstein and Bloom, 2005). In the synthesis process of the secondary metabolite, the role of nitrogen as a building material, especially for synthesis of amino acid (tyrosine and phenylalanine) through biosynthetic pathway of shikimic acid inform of phenile propane (C6-C3) (Winkel, 2006).
Relationship between N-total in the soil and vitexicarpine concentration in the young and old leaves of V. trifolia L was presented at Figure 4. Based on the Figure 4, nitrogen in the soil has a positive correlation with vitexicarpine concentration in the leaves of V. trifolia L.
It is well known that N is a mobile nutrient in the plant, so the N content in the young leaves is higher than the olderone (Epstein and Bloom, 2005). This has become one of the causes of vitexicarpine concentration in young leaves was higher than in older leaves. Concentration of N in Entisols and Inceptisols were observed at range of 0.06-0.09%. According to soil chemical analysis criteria (SRC, 2009), the range of the value could be categorized as a very low rate, while in Andisols were observed at range of 0.25-0.38 (medium) (Table 1).
Magnesium (Mg) also an important macronutrients required by plant as a nucleus of chlorophyl molecule. The molecule has an important role in photosynthesis, synthesis of fatty acid and oil in the plant (Epstein and Bloom, 2005). In the synthesis of secondary metabolite, the role of Mg as a catalyst for biosynthetis pathway from malonic acetate to Acetyl CoA (C2) and contributed in increasing the vitexicarpine in the leaves of V. trifolia L. Combination of the both biosynthetic pathway of malonic acetate and shikimic acid resulted in flavonoid compounds. The flavonoid compound should undergo a flavonoid methyltransferase process with Mg2+ ion and SAM as catalyst and finally resulted in the vitexicarpine compound (Torsell, 1997). Relationship between Mg-available in the soil and vitexicarpine concentration in the young and older leaves of V. trifolia L. was prsented in Figure 5. Based on the figure, magnesium in the soil has a positive correlation with vitexicarpine concentration in the leaves of V. trifolia L.
Magnesium is a mobile nutrient in the plant even not as mobile as nitrogen, but Mg in the older leaves will be translocated to the young leaves. That is why the Mg content in the young leaves is higher than the older one (Epstein and Bloom, 2005). This may be related to the positive correlation between Mg and vitexicarpine concentration in the leaves of V. trifolia L. and also resulted in the vitexicarpine concentration in young leaves was higher than in older one.
Concentration of Mg in Andisol, Entisols and Inceptisols were observed at range of 2.21-9.67, 13.34-14.44 and 10.57-13.74%, respectively. According to soil chemical analysis criteria (CSR, 2009), the range of the value could be categorized as a high-very high rate. A high availability of Mg in the soil resulted in the Mg content in the plant also become high and may stimulate for formation of Clorophyl in turn, the production of oil fatty acid and metabolite secondary (such as vitexicarpine) also increased (Marschner, 1995; Merhaut, 2007).
A negative correlation was observed for the soil quality index and vitexicarpine content in the leaves of V. trifolia. Lower the soil quality tends to result in higher content of vitexicarpine. Soil quality index of Andisols from Karangpandan-Surakarta was 0.991 and vitexicarpine content in the leaves was only 0.6%, but in case of Entisols was 0.274 and vitexicarpine content was able to reach around 0.82%. Inceptisols quality index was an intermediate between Andisols and Entisols. However, individually nutrient indicated a positive correlation with vitexicarpine content. Higher Nitrogen and Magnesium content in the soil resulted higher vitexicarpine content in the leaves. This may related to the role of the both nutrient in biosynthesis of vitexicarpine.