ABSTRACT
This study was conducted to evaluate the effect of urea and molasses addition on physical properties, pH, temperature, dry matter loss and chemical composition of sugarcane top (SCT) silage. Treatments were arranged as 2*4 factorial, with two SCT types (green and burnt) and four silage treatment types (without additive, 4% molasses, 1% urea and 1% urea + 4% molasses) in a completely randomized design. Forages were chopped into 2 to 3 cm, treated with the additives and ensiled in 1.09 L mini silos for 45 days. The best average score values for smell, color, texture, mold appearance and pH was noted in silages made without additive or with molasses. The desirable pH (3.7 to 5.0) was obtained in all silages, except in green SCT ensiled with urea-based additives, while lower (P<0.0319) total dry matter loss (2.31%) and temperature (26ËšC) were noted in green SCT ensiled with molasses alone. Silage protein content increased (P<0.0001) with urea addition, but not (P>0.05) with molasses alone. Fiber fractions (NDF, ADF and ADL) of burnt SCT were not affected (P>0.05) by additives, while NDF increased with urea based additives and ADF decreased with molasses based additives in green SCT silage. The highest (P<0.0001) in vitro dry matter digestibility (53.68%), organic matter digestibility (48.34%) and metabolizable energy (7.74 MJ/kg DM) content were observed contained in burnt SCT silage treated with urea-molasses mix, whereas a significant reduction (P<0.0001) in non-fiber carbohydrate content was observed in green SCT ensiled with urea (8.4%) and urea + molasses (1.65%). In conclusion, both burnt and green SCT can be adequately fermented and preserved as silage without additive; however silage nutritive value, particularly of burnt SCT can be further enhanced by ensiling with urea and molasses.
Key words: Sugarcane top, molasses, urea, silage.
Feed deficit is a critical bottleneck to livestock production in Ethiopia. The root causes are shrinkage of natural grazing land, underdeveloped forage production, low availability and poor quality of feeds, high cost of feeds and frequent drought occurrence. However, there are potentially available unconventional feedstuffs that if properly exploited, can support livestock production. Sugarcane top (SCT) is one of such feed resources largely available at sugar factories and in private farms in Ethiopia. At factory level, it is often available in burnt form, after cane harvesting, representing 15 to 25% of plant biomass (Suttie, 2000) or 25 to 30% of cane yields that is equivalent to 5 to 6 tons DM per hectare. Recently, a number of new sugar factories are emerging in Ethiopia, generating large amount of sugarcane top for livestock feeding mainly for farms close to the factories. Also, over 31,236 hectares of land are covered by business-oriented private cane plantation (CSA, 2017) of diverse germplasm and production potential (Tena et al., 2016), which significantly contributes to farm level green SCT production. Sugarcane top is highly palatable with good intake characteristics for livestock (Suttie, 2000). It is comparable with average quality grass hay, but deficient in protein, mineral and energy (Leng and Preston, 1985). A notable problem with SCT is that it loses quality through time, becoming rough and less palatable to animals during drying and storing. When stored for longer period, it forms mold and deteriorates in quality. Studies have shown that ensiling is a possible means of conserving SCT (Siqueira et al., 2009; Akinbode et al., 2017). .Sugarcane top is rich in water soluble carbohydrate (155 g/kg DM (Khanal et al., 1995), 82.5 g/kg DM (Chaudhry and Naseer, 2008)), which is of a desirable characteristic for successful ensiling. Therefore, fresh SCT silage making might have a comparative advantage over hay making for ruminant livestock feeding. Application of additives in silage making is one of the management practices crucial at ensiling time, storage and feed-out phase to reduce nutrient loss and improve its nutritive value. Various chemical and biological additives have been used to control undesirable microorganisms (e.g, aerobic bacteria and fungi) and improve aerobic stability in silages (Pedroso et al., 2008; Pedroso et al., 2011; Siqueira et al., 2011). The most useful additives are molasses, which is a source of fermentable carbohydrate, and urea that provides fermentable nitrogen for microorganisms in the silage and rumen of the animal. Also, urea has buffering capacity by raising the pH of the silage at early stage of fermentation that might inhibited yeast growth. Various studies have illustrated the beneficial effect of urea and/or molasses applications in silage making (Suárez et al., 2011; Kaensombath and Lindberg, 2013; Kung and Shaver, 2001; Khanal et al., 1995; Tadesse et al., 2014). In Ethiopia, surplus SCT is often available during dry season when cane harvesting is practiced and green fodder for livestock feeding is most limited. Therefore, a proper conservation practice to optimize its use for livestock feeding has to be investigated. Moreover, limited studies have been done on SCT silage manufacture in the country. Hence, this study was aimed to evaluate the role of urea and molasses application on physical properties, fermentative quality and nutritive value of burnt and green SCT silages.
Experimental site
The experiment was conducted at Debre-Zeit Agricultural Research Centre (DZARC), livestock research farm, located at 45 km southeast of Addis Ababa (08°44'N latitude, 38°58'E longitude; altitude of1900 m above sea level). The area is known for bimodal rainfall, with average annual rainfall of 814 mm and minimum and maximum temperature of 10.9 and 28.3°C, respectively (DZARC, unpublished data).
Treatments and design
The treatments were set in a 2 x 4 factorial arrangement of treatments (2 sugarcane top forms (green and burnt) and 4 silage treatment types (no additive, 4% molasses, 1% urea, and 1% urea + 4% molasses) in a completely randomized design. A polyethylene container (“mini silo”) with a volume of 1.09 L was used in 5 replicates per treatment, making a total of 40 experimental silos. The additives were added on the basis of forage dry matter. Fertilizer grade urea was used for this purpose. The levels of application were adopted from research reports (Suárez et al., 2011; Khanal et al., 1995).
Sampling and ensiling procedure
Sugarcane top of mature cane (variety N-14, or Natal) were collected from Wonjishoa sugar factory plantation before and after burning at harvesting. The cane field was often burnt to ease the harvesting practices and add ash to the soil. The sugarcane was grown on heavy black soil (Vertisols), aged 23 months and harvested at 1st stage of cutting after planting. Sampling was done randomly at six marked specific sites (at equal interval) along the gradient line (diagonally and horizontally) in one hectare cane field. The green SCT were sampled overnight before burning, while the burnt samples were taken in the next morning from the same site. The cutting point for SCT sampling was used by staff (cutters) of the sugar factory for cane harvesting. Immediately after cutting, the respective samples were put into polyethylene sheet and transported to the research center. After arrival, the burnt and green samples were mixed thoroughly and independently, chopped using electrically operating machine (Ethio-chopper, Ethiopia) into 2 to 3 cm length. The amount of chopped SCT (green/burnt) used for ensiling was weighed and wilted under shade, to attain about 35% DM for both burnt and green SCT (Table 5). Urea was diluted with water at a ratio of 1:1.5 when used as sole additive. When molasses alone, or urea and molasses were mixed, the amount of water used for dilution equaled the amount of molasses used by weight (Suárez et al., 2011). The chopped materials were weighed, except for the control all were thoroughly mixed with the respective additive on polyethylene sheet laid on concrete floor. The forages were filled into the mini silos lined inside with polyethylene sheet. All silos were filled at similar packing density (767 g per 1.09 L plastic bottle) by hand filling and pressing with a wooden stick. The tightly packed silos were immediately closed, tightly sealed and placed under shade which is allow to ferment for 45 days at room temperature. Adequate samples of the respective untreated and treated materials were taken at ensiling, put in polyethylene bags, sealed and stored in deep freezer (-20°C), awaiting for laboratory analysis.
Silage temperature and pH
On day 45 of ensiling, all silos were weighed. Temperature after opening each silo was measured using a laboratory thermometer inserted into silo’s center. After observation for mold occurrence, the silages were removed, homogenized and sampled in two parts where one part was immediately frozen, while the remaining was used for physical characteristics assessment. About 20 g of frozen silage sample per treatment was taken in a beaker to which 100 ml of distilled water was added (AFIA, 2011). The samples were blended using a glass stirrer and left for 1 h before filtering with filter paper. Silage pH was measured from the extract using a conventional digital pH meter (Hanan Bench top pH meter), calibrated with buffer solutions (pH 4 and 7).
Visual appraisal of silage quality
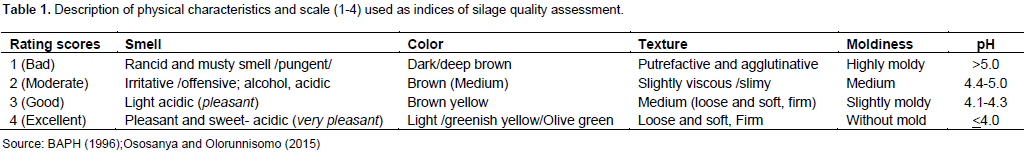
The contents of the silos were evaluated for physical attributes by a panel discussion involving six trained personnel on the indices and scales of silage quality characterization. The panelists were all from the Department of Livestock Research with different professional background, but had experience on silage making. They were trained on how to apply the criteria set (subjective score 1-4; Table 1) and exercised them before commencing the actual evaluation, independently. Observation for mold formation was done starting from the silo opening time, while color, smell and texture were evaluated after silo content extraction. The visual observation for color assessment was also aided by standard color charts. The score values of each individual for all attributes were used in the statistical analysis.
Chemical analyses of samples
Sugarcane top samples (intact green, burnt and silages) were dried in a forced air oven at 60°C for 72 h and ground to 1.0 mm size in a Wiley mill. For all silage samples, dry matter (DM), crude protein (CP=N * 6.25), ash, ether extract (EE), calcium (Ca) and phosphorus (P) contents were determined according to AOAC (1990), while neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) contents were analyzed according to Van Soest and Robertson (1985). The in-vitro organic matter and dry matter digestibility coefficients (IVOMD and IVDMD) were determined according to Tilley and Terry (1963) by applying a two-stage digestion process, in which samples were first fermented in rumen fluid obtained from donor animals, followed by acid digestion. Total dry matter loss (TDML) was calculated by DM weight loss in the silage ((DM of forage -DM of silage)/DM forage*100) (Pedroso et al., 2011), Non-fibre carbohydrate by (% NFC) = 100–% (CP + Ash + EE + NDF) (Hall, 2000) metabolizable energy by (ME, MJ/kg DM) = IVDOMD (g/kg DM) *0.016 (McDonald et al., 2010) and Hemicelluloses = NDF-ADF.
Statistical analysis
The data were subjected to analysis of variance using General Linear Model procedure of SAS program (SAS, 2004). When interaction between factors was non-significant, only main effect means were presented and discussed, otherwise simple effect means were presented. Mean separation was done using Tukey test at 5% probability. The statistical model used was: Yij = µ + αi + Tj + (αT)ij + εij; where; Yij is the response variable; m = Overall mean, αi = the effect of cane top type (i = burnt and fresh); Tj = Effect of silage treatment type (j = ± additive), (αT)ij = Interaction effect of cane top type "i" with silage treatment type "j" and εijk = the experimental error.
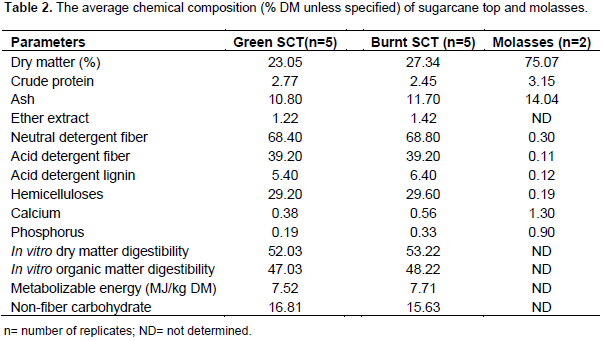
Chemical composition of SCT and molasses
The chemical composition of sugarcane top and molasses is indicated in Table 2. The burnt and green SCT did not differ markedly in nutrient contents. However, burnt SCT had slightly lower contents of CP and NFC and higher in other parameters, except ADF, than the green SCT. The losses of moisture and some organic matter, occurred during burning probably elevate the concentration of some nutrients in burnt SCT. Similar to the present finding, a slight increase in DM, ash and hemicelluloses, but reduced NDF, ADF and ADL were reported in burnt than green SCT (Magaña et al., 2009). On the other hand, Ramírez-Cathí et al. (2014) researched on green and burnt SCT of four mature sugarcane varieties and found no difference (P>0.05) in DM, CP, NDF, ADF, ash and in-vivo digestibility values among varieties and harvest types. The present average CP content of green SCT was lower than the reported values by other studies (Akinbode et al., 2017; Sharma et al., 2012; Khanal et al., 1995). However, Sharma et al. (2012) stated that various green SCT varieties when sampled at 3rd stage of cutting had a CP content as low as 1.5%, while other researchers reported a level ranged from 2.5 to 3.6% CP (Tadesse et al., 2014; Ramírez-Cathí et al., 2014; Anteneh, 2014). The variation in CP values may be associated with the differences in varieties, stage of growth and/or nitrogen fertilizer application. Nevertheless, all of them had CP values below the optimum level for rumen fermentation. Similarly, contents of the fiber components (NDF, ADF and ADL) and EE were within the range of values reported in previous studies (Tadesse et al., 2014; Sharma et al., 2012; Gendley et al., 2002). However, significantly higher ADL contents (14%DM) of green SCT than the present results were reported by Akinbode et al. (2017) and Anteneh (2014). Hemicelluloses and P content of green SCT in the present study were close to the values reported by Gendley et al. (2002).
Silage pH, physical properties, temperature and dry matter loss
The pH of pre-ensiled materials (treated/ untreated SCT) was higher (P< 0.05) for sole urea treated SCT compared to untreated one (control), but not varied among other treatments (Table 3), partly indicating the buffering effect of urea even at the initial stage. Regardless of the treatments used, initial pH was higher (P=0.0025) in green than in burnt SCT. The pH of SCT after 45 days of fermentation was affected by the interaction of treatment and SCT type (P<0.05); with the burnt SCT being unaffected by treatment while values for urea containing treatments were higher as compared to untreated and 4% molasses treatments in green SCT silage. On the other hand, except for SCT treated with urea and molasses combination where the pH was lower for the burnt SCT, the pH of the burnt and green SCT were similar for the other three treatments containing urea. The results signify that, both the green and burnt SCT has adequate soluble sugar for satisfactory fermentation. Similarly, low pH value of SCT silage ensiled without additive was reported (Khanal et al., 1995). Furthermore, Heikkilä et al. (2010) reported that, wilting pre-ensiled grass to DM content above 30% restricted the extent of fermentation resulting in well-preserved silage at no use of additive. Except for green SCT that ensiled with urea containing additive, the rest of the silages had pH within the range 3.7 to 5.0 acceptable for good to average quality silage (McDonald et al., 2010). The drop in pH at the end of ensiling from the initial value is presumably a consequence of production of organic acids (McDonald et al., 1991). While molasses was exclusively added, silage pH was similar to that of control, probably indicating that SCT had the threshold soluble carbohydrate to trigger the production of lactic acid.
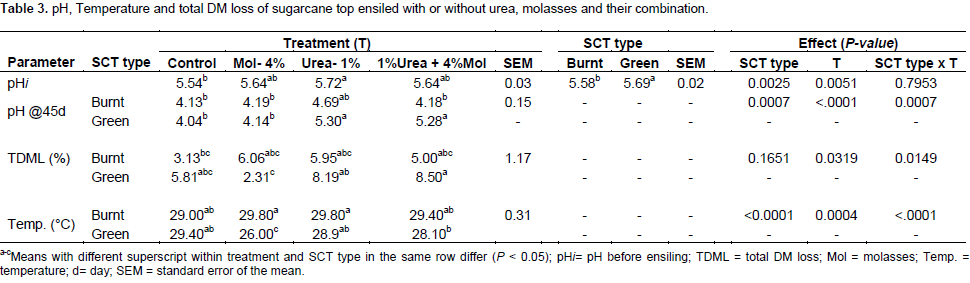
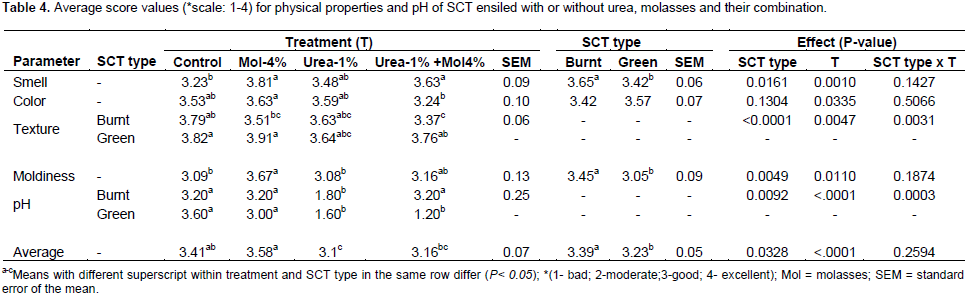
However, when SCT was ensiled with urea exclusively, the levels of pH were significantly increased (P<0.05) over that of control silage especially for green SCT above the recommended pH 4.5, indicating that urea might have reduced fermentation and acid production during ensiling. During ensilage, the ammonia released from urea is slightly basic, causing delay in pH drop and DM loss in grass silage (Kung and Shaver, 2001). Molasses when used in combination with urea failed to significantly counter-act the effect of urea alone to reduce silage pH both in green and in burnt SCT silages. However, Khanal et al. (1995) reported a significantly reduced pH of green SCT silage treated with urea and molasses combination when compared with urea only treated with green SCT silage. Generally the application of silage additives used in the current study had no beneficial effect in reducing total dry matter loss. Similar to the result of the current study, increased pH were observed in green SCT (Khanal et al., 1995) and Napier grass (Samanta et al., 2001) ensiled with urea and urea-molasses as additive, leading to poor fermentation of silage. Total dry matter loss was lower (P<0.05) in green SCT ensiled with molasses alone than the other additive containing treatments. Paderoso et al. (2011) reported 31% reduction in dry matter loss of sugarcane silage when ensiled with urea at lower dose (0.5%) than the present dose. Treating green SCT with urea alone resulted in three-fold TDML over molasses alone in silages (2.31 vs. 8.19), implying that undesirable microbes might have been favored by urea than molasses addition. The temperature of silages measured upon opening the silos, ranged from 26 to 29.8°C. Value were similar among treatments for the burnt SCT, while for green SCT, the temperature of molasses alone treated silage was lower than the silage treated with combination of molasses and urea. The present silage temperatures of SCT ensiled without additive which exceed the value of 26°C as reported by Akinbode et al (2017) after 42 days of ensiling. Temperature range from 27 to 32°C is often acceptable as indicator of good fermentation status of silage. Kung (2011) suggested that, silage temperature of small silos similar to the ambient temperature or just a few degrees warmer is normal. Thus, the result of this study showed that the generated heat was small, indicating the occurrence of minimal aerobic deterioration. Excessive heat production due to aerobic oxidation leads to browning (Millard) reaction, forming protein and carbohydrate complex that inhibits protein and fiber digestion (Bolsen et al., 1996). There was no significant interaction between treatment and SCT forages, except for texture and pH, on the average score values of silages (Table 4). The average score value for smell increased (P<0.001) for molasses containing additives when compared to the control, and was higher (P<0.0025) in burnt than the green SCT silage. The score for color was lower in silages treated with molasses + urea than with molasses alone. Score for texture was higher (P=0.0047) in molasses containing treatments of the green SCT than burnt SCT silages, and among the treatments differences were observed between control and molasses+urea containing treatments for the burnt SCT. Moldiness score increased in molasses based additives, and was higher in burnt than green SCT silages. Generally, a slight mold was observed on top of all silos, which could be due to air trapped while sealing. The score of pH was lower for urea treated silages than other treatments. Accordingly to the scale used in this study, the average score values of the silages indicated that the best physical attributes and pH values were attained when SCT was ensiled without additive or with 4% molasses alone. Akinbode et al. (2017) reported that green SCT ensiled without additive which had a greenish yellow color, pleasant smell and slightly moldy.
Change in dry matter and nutrient composition
There was a significant interaction between treatments and sugarcane top types (P<0.05) on chemical composition of silages except for ether extract (Table 5). For burnt SCT molasses + urea, additive treatment was higher than the other treatments, while for green SCT molasses + urea additive treatment had the highest and the control had the lowest DM content of pre-ensiled forages. Addition of molasses increased (P<0.05) DM content of fermented green SCT silages as compared with control silage, or that treated with urea alone. Generally there was a reduction of less than 4.6% in DM content of the ensiled material as compared to pre-ensiled SCT, presumably due to loss of soluble nutrients during fermentation. McDonald (2010) reported that up to 5% DM loss occurring during ensiling process is considered as normal. Addition of molasses alone do not increase (P>0.05) CP content when compared with control silages. However, SCT ensiled with urea based additives had significantly higher (P<0.05) CP content than the treatments without. These results are in agreement with reports of Pedroso et al. (2011), where urea addition increased CP content of sugarcane silage. In contrary, Khanal et al. (1995) reported that no difference in CP content between control and urea treated green SCT silages, but increased when molasses was included at 3 to 12% DM. The NDF content increased with urea based additives, while ADF decreased with molasses based additives in green SCT. For burnt SCT, NDF and ADF values for molasses + urea additive silage was lower than urea alone additive silage. This could be possibly due to low acid condition of urea treated silages, resulting in reduced NDF solubility and/or increased loss of soluble nutrients. With increase in silage acidy a decrease in NDF content was reported (McDonald et al., 1991). The increase in fiber components of SCT silages associated with urea based treatments could be due to loss of some fermentable carbohydrates, leading to rise in the concentration of NDF in DM. The application of molasses alone lowered NDF in green than burnt SCT silage, while the reverse is true with urea and molasses combination. This can be explained from reduction in non-fiber carbohydrate (NFC) for green SCT silages treated with urea or, urea + molasses than the corresponding burnt SCT silages. The loss in soluble sugar arises from microbial fermentation which occurs during ensiling (McDonald et al., 1991).
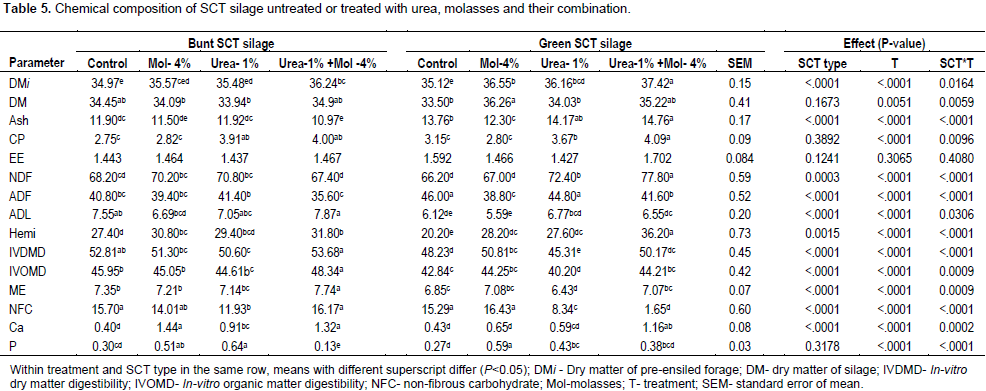
The in vitro dry matter digestibility (IVDMD) and organic matter digestibility (IVOMD) were lowest (P<0.0001) in green SCT ensiled with urea alone. Except for molasses treated silages, higher digestibility coefficients were obtained in burnt than green sugarcane top silages. The highest IVDMD (P<0.0001) and IVOMD values were obtained in burnt SCT ensiled with urea+molasses, which could be related to lower fiber contents in this treatment (NDF, ADF and hemicelluloses). In agreement with the present finding, Khanal et al. (1995) reported increased IVDMD and IVOMD of green SCT silage ensiled with urea + molasses when compared to silage without additive, but addition of urea had no effect over control. The highest ME value was noted in the burnt SCT silage treated with urea + molasses, and the lowest was in green SCT ensiled solely with urea. The ME value of intact SCT was reported to be 7.0 MJ/kg DM (McKenzie and Griffiths, 2007). Higher NFC value was obtained in green SCT ensiled without additive, or with molasses alone compared with urea based treatments. In green SCT silage, this value was low when ensiled with urea and largely depleted with urea + molasses treatment. Akinbode et al. (2017) reported that, green SCT ensiled without additive had 13.9% NFC after 42 days of ensiling, which approached the green SCT (15.3%) in the present study. The higher value of NFC indicated that the silages were well fermented and preserved. Moreover, Ferreira et al (2014) reported that, fermentation characteristics of elephant grass low in NFC (3.2%) was greatly improved by addition of cashew bagasse rich in NFC (11.9%). Ether extract values of the silages were not affected by SCT types and treatment (P>0.05).
Ensiling SCT with urea and molasses had beneficial effect on the fermentative quality and nutritive value of SCT silage. In terms of digestibility, green SCT was best fermented and preserved when ensiled without additive or treated with 4% molasses, while the burnt SCT was best preserved with 1% urea and 4% molasses combination. It can however be established that, both burnt and green SCT can be adequately fermented and preserved as silage without additive like urea and molasses and their combination.
The authors have not declared any conflict of interests.
Getahun Kebede would like to thank Ethiopian Institute of Agricultural Research for financing this study, and the technical and research staff in the Department of Livestock Research and Nutrition Laboratory of Debre-Zeit Agricultural Research Center for their support in implementing the experiment. The author also acknowledged Wonji- shoa sugar factory for permitting access to cane plantation and SCT sampling.
REFERENCES
|
Australian Fodder Industry Association (AFIA) (2011). Laboratory Methods Manual. Version 7:80-81.
|
|
|
|
Akinbode RM, Isah OA, Oni AO, Arigbede OM, Ojo VOA (2017). Nutritional evaluation of sugarcane top ensiled with varying proportion of broiler litter. Livest. Res. Rural Dev. 29:6.
|
|
|
|
|
Anteneh W (2014). Effect of Different Levels of Dried Sugar Cane Tops Inclusion on the Performance of Washera Sheep Fed a Basal Diet of Grass Hay. A Thesis Submitted to the School of Graduate Studies (School of Animal and Range Sciences), Hramaya University, Ethiopia.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (1990). Official Methods of Analysis. 15th Edition. Arlington, Verginia, USA. pp. 12-98.
|
|
|
|
|
Bolsen KK, Ashbell G, Weinberg ZG (1996). Silage fermentation and silage additives. Rev. Asian-Aust. J. Anim. Sci. 9:483-493.
|
|
|
|
|
Bureau of Animal Production and Health (BAPH) (1996). Criteria for evaluation of maize stover silage. BAPH, Ministry of Agriculture, Bejing, China. FAO publication.
|
|
|
|
|
Central Statistics Agency (CSA) (2017). Agricultural sample survey, Area and production of major crops: private peasant holdings. Ethiopia: Stat. Bull. 1:584.
|
|
|
|
|
Tena E, Mekbib F, Shimelis H, Mwadzingeni L (2016). Sugarcane production under smallholder farming systems: Farmers preferred traits, constraints and genetic resources. Cogent Food Agric. 2:1191323.
Crossref
|
|
|
|
|
Gendley MK, Singh P, Garg AK (2002). Performance of Crossbred Cattle Fed Chopped Green Sugarcane top and Supplemented with Wheat Bran or Lentil Chuni Concentrates. Asian-Aust. J. Anim. Sci. 15(10):1422-1427.
|
|
|
|
|
Hall MB (2000). Neutral detergent-soluble carbohydrates. Nutritional and relevance analysis. Florida: University of Florida. Bulletin P. 339.
|
|
|
|
|
Heikkilä T, Saarisalo E, Taimisto AM, Jaakkola S (2010). Effects of dry matter and additive on wilted bale silage quality and milk production. Grassland Sci. Eur. 15:500-502.
|
|
|
|
|
Kaensombath L, Lindberg JE (2013). Effect of additives on ensiling of taro (Colocasia esculenta (L.) Schott) leaves and Stylo CIAT 184 (Stylosanthes guianensis (Aubl.) Sw. var. guianensis) forage. Livest. Res. Rural Dev. 25:69.
|
|
|
|
|
Khanal RC, Perera ANF, Perer ERK (1995). Ensiling characterstics and nutritive value of sugarcane top. Trop. Agric. Res. 7:177-185.
|
|
|
|
|
Kung L (2011). Silage Temperatures: How Hot is Too Hot? Dept. of Animal & Food Sciences. 531 South College Avenue, Newark, DE 19717-1303.
|
|
|
|
|
Kung L, Shaver R (2001). Interpretation and use of silage fermentation analysis reports. Focus on Forage 3(13):1-5.
|
|
|
|
|
Leng RA, Preston TR (1985). Constraints to the efficient utilization of sugarcane and its by-products as diets for production of large ruminants. In: R M Dixon (ed.), Ruminant feeding systems utilizing fibrous agricultural residues. International Development Program of the Australian Universities and Colleges, Canberra, Australia pp. 27-48.
|
|
|
|
|
Maga-a R, Aguirre J, Aguirre A, Gómez A, Martínez S, Lemus C, Ulloa R, Ly J (2009). Entire sugar cane or sugar cane residues for feeding sheep. Performance traits of hairless sheep. Livest. Res. Rural Dev. 21:24.
|
|
|
|
|
McDonald P, Edwards RA, Greenhalgh JFD, Morgan CA, Sinclair LA, Wilkinson RG (2010). Animal Nutrition. 7th Ed. Ashford Color Press Ltd., Gosport, England.
|
|
|
|
|
McDonald P, Henderson AR, Heron SJE (1991). The Biochemistry of Silage. 2nd Ed. Chalcombe publications, Marlow, Bucks, UK.
|
|
|
|
|
McKenzie J, Griffiths C (2007). Cane tops as cattle fodder. New South Wales Department of Primary Industries, Primefacts, N°314.
|
|
|
|
|
Ososanya TO, Olorunnisomo OA (2015). Silage characteristics and preference of sheep for wet brewer's grain ensiled with maize cob. Livest. Res. Rural Dev. 27:12.
|
|
|
|
|
Pedroso AF, Rodrigues AA, Júnior WB, Souza GB (2011). Fermentation parameters, quality and losses in sugarcane silages treated with chemical additives and bacterial inoculants. R. Bras. Zootec. 40(11):2318-2322.
Crossref
|
|
|
|
|
Pedroso AF, Nussio LG, Loures DRS (2008). Fermentation, losses, and aerobic stability of sugarcane silages treated with chemical and bacterial additives. Sci. Agric. 65:567-691.
Crossref
|
|
|
|
|
Ramírez-Cathí H, Aida C, Salcedo C, Briones F, Lucero FA, Cárdenas A, Marcof C, Martínez JC (2014). Bromatological and morphological characterization and yield of sugar cane top in Huasteca Potosina, Mexico. Cuban J. Agric. Sci. 48:4.
|
|
|
|
|
Samanta AK, Singh KK, Verma NC, Misra AK (2001). Effect of additives on silage quality of Napier ensiled in plastic bag. Indian J. Anim. Sci. 71(9):881-882.
|
|
|
|
|
Statistical Analysis System (SAS) (2004). Version 9.1. SAS Institute. Inc., Cary, NC, USA.
|
|
|
|
|
Sharma VK, Tomar SK, Kundu SS, Pankaj J, Pankaj J, Muneendra K, Manju L (2012). Chemical Composition and Effect of Feeding Different Levels of Sugarcane top with Concentrate Mixture/Mustard Cake on Digestibility in Buffalo Calves Indian J. Dairy Sci. 65:5.
|
|
|
|
|
Suárez R, Mejía J, González M, García DE, Perdomo DA (2011). Evaluation of mixed silages of Saccharum officinarum and Gliricidia sepium using additives. Pastos Forrajes 34(1):69-86.
|
|
|
|
|
Suttie JM (2000). Hay and straw conservation for small-scale farming and pastoral conditions. FAO Plant Production and Protection Series No. 29, FAO, Rome.
|
|
|
|
|
Tadesse A, Fulpagare YG, Gangwar SK (2014). Effect of urea treatment on chemical composition and oxalate content of sugarcane top. Int. J. Sci. Nature 5(1):15-18.
|
|
|
|
|
Tilley J, Terry RA (1963). A two stage technique for the in-vitro digestion of forage crops. J. Brit. Grassland Soc. 18:104–111.
Crossref
|
|
|
|
|
Van Soest P, Robertson JB (1985)s. analyses of forage and fibrous foods. A laboratory manual for Animal Science 613. Cornell University, Ithaca, New York, USA.
|
|