Full Length Research Paper
ABSTRACT
Maize (Zea mays L.) is a widely cultivated crop in South Africa and forms the main food crop of thousands of rural communities in the country. In order to improve food and nutrition security for marginalised communities, there is need to develop numerous elite quality protein maize (QPM) varieties. The success of a breeding programme is dependent on the existence of molecular variability among the germplasm. The diversity within 45 QPM inbred lines was evaluated using simple sequence repeat markers. Twenty seven simple sequence repeat primers amplified a total of 112 fragments among the inbred lines. The mean polymorphism information content was 0.48, with an average of 4.32 alleles per locus. Cluster analysis using Rogers (1972) genetic distance partitioned the inbred lines into two major clusters with four and nine sub-clusters each. The minimum genetic distances was 0.13 between CIM12 and CIM13, the average genetic distance was 0.32 and the maximum was 0.46. Cross combinations between QS1 and CIM19 and those between QS22 and CIM18 can potentially give substantial heterosis because of the moderate (0.46) genetic distances that were found between them. Hybrids between these parental lines need to be generated and evaluated in yield trials.
Key words: Quality protein maize, diversity, inbred lines.
INTRODUCTION
Maize is the most important grain crop in South Africa and plays a vital role especially in the diet of women, children, weaned babies and the sick in marginal rural areas of the Eastern Cape Province. It provides food security and a means of livelihood to the majority of people in the province who depend on the crop for daily calories and nutrients. Just like most crops, normal maize provides the recommended calorie amounts but it does not meet all the nutrient requirements of the human body. Quality protein maize (QPM) is a type of maize that contains nearly twice the amount of lysine and tryptophan found in normal endosperm maize.
The benefits of consuming QPM far exceed those of normal maize. QPM has a biological value of protein of 80% compared to that of milk which is 90%, while that of normal maize is 45%. The biological value is an indication of the amount of nitrogen that can be absorbed for synthesis of amino acids required for the various metabolic functions in the body (Prassana, 2001). QPM is a nutrition smart food crop providing an improved quality of protein especially for communities who cannot afford protein supplements. Additional benefits of QPM include higher protein retention in people, less sick days for infants, and a quick recovery time for malnourished children. When maize consumption rates of QPM were compared to those of normal maize, it was revealed that children need to consume 100g of QPM to achieve the daily recommended protein requirements compared to 500 g of normal maize (Nuss and Tanumihardjo, 2011). This means that, a harvest of QPM maize will go a longer way in providing nutrition than that of normal maize. Quality protein maize feeding trials reported a 60% increase in live weight for pigs fed on QPM compared to those fed on normal maize. The use of QPM for animal nutrition has resulted in farmers purchasing less soybean and fish meals as protein supplements and using the money for other inputs.
The development of QPM began in the 1970’s in South Africa’s KwaZulu Natal Province. However, its adoption and utilization has been remarkably low in South Africa and neighbouring countries (Van de Merwe, 1995). Due to the high dependency on maize by rural communities, it has become imperative to identify quality protein maize inbred lines that can be used in breeding programs to develop competitive hybrid and synthetic varieties. Only twelve white QPM hybrids and five QPM open pollinated varieties have been developed and registered in South Africa compared to more than one hundred normal maize varieties. Eighty eight percent of the QPM varieties available were developed by Quality Seed cc (QS) (DAFF, 2014). However, these varieties were bred mainly for high potential areas in the KwaZulu Natal Province (Dr Gevers, personal communication). On the other hand, the Eastern Cape’s climate is characterized by a high evaporative demand, erratic rainfall and high summer temperatures (Van Averberke et al., 2011) not ideal for the existing QPM varieties.
With no more arable land available and the demand for agricultural produce continuously increasing, crop improvement can play a significant role in ensuring sustainable food security for marginal areas. However, the basis of crop improvement involves harnessing the variability among germplasm, which will facilitate selection of potential genotypes with potential for producing maximum heterosis when used in crosses. Detailed knowledge on the available QPM inbred lines is required for them to use them efficiently in breeding programmes.
At present no information is available on the genetic diversity between CIMMYT and QS QPM inbred lines. Therefore, determination of the genetic relationships of germplasm from these two sources would be of interest to maize breeders targeting the development of highly productive QPM cultivars not only for the Eastern Cape province of South Africa but for Southern Africa. Genetic diversity can be investigated using several techniques such as morphological or molecular markers.
Molecular markers can reveal genetic relationships among the inbred lines. Crosses between inbred lines that are genetically distant are expected to give a larger genetic variance among progenies than crosses between closely related lines (Hallauer and Miranda, 1988). Molecular markers have been extensively used in maize genetic studies for the analysis of genotype frequencies, identification of deviations at individual loci and for characterization of molecular variation within and between populations. Relative to other types of molecular markers, simple sequence repeats (SSRs) are technically simple to use, cost effective, co-dominant, robust and reliable, and they are transferrable between populations (Collard et al., 2008).
According to Shin et al. (2006), genetic distance measurement ensures a better understanding of the genetic structure and helps in genetic manipulation of genotypes for crop improvement. Genetic distances are estimated from assessment of genetic diversity between genotypes. Mondini et al. (2009) defined genetic diversity as the variety of alleles and genotypes present in a population, which is reflected in morphological and physiological differences between individuals of a population. Knowledge of the genetic distance of inbred lines enables those from different heterotic groups to be combined to form a heterotic pattern. Heterotic patterns can be used in selecting parents of crosses for line development. In addition the heterotic patterns can be used in selecting testers for evaluating the combining abilities of new inbred lines.
Maize breeders are also interested in selecting inbred lines that combine well and give high yields without necessarily making all possible crosses between them (Makumbi et al., 2011). In the present study, assessment of genetic diversity was of interest for broadening the current QPM genetic base. The highest genetic distance reported among CIMMYT-QPM inbred lines used in this study was 0.38 (Pfunde, 2013), which necessitated broadening the current QPM genetic base by including those from QS cc. The objective of this study was therefore to assess the genetic diversity among 45 QPM inbred lines using SSR markers.
MATERIALS AND METHODS
Forty-five white grained QPM inbred lines were sourced from CIMMYT –Zimbabwe (CIM) and Quality Seed cc (QS) in KwaZulu Natal Province, South Africa. The QPM inbred lines are described in Table 1.
Genetic diversity using SSR analysis
Quality protein maize inbred lines were planted in pots in a glasshouse at the University of Fort Hare in February 2014. Maize genomic DNA was extracted from 2 week old leaves from each of the 45 QPM inbred lines. Extraction was carried out using a Wizard® genomic DNA purification kit (Promega) from 40 mg of maize leaf tissue that was freeze dried using liquid nitrogen.
In order to determine the quality of DNA, 2 µl of concentrated DNA sample was mixed with 10 µl of 6x loading dye. The mixture was loaded on a 0.8% agarose gel, and electrophoresis was carried out in a buffer with 0.5 Tris Borate Ethylenediaminetetraacetic acid (TBE) with a pH of 8.0, using a Gel XL Ultra horizontal gel system (Labnet International) at 100v for 90 min. A 1 kb ladder was used as the molecular weight marker. After electrophoresis, the DNA was stained with ethidium bromide and then visualised using a gel documentation system (Uvitec Cambridge, Alliance version 4.7). The quantity of DNA was determined by Ultraviolet absorbance using a spectrophotometer (Genova MK3 Life analyser, Jenway). For quantity assesement, 5 µl of the concentrated DNA sample, plus 995 µl of Tris EDTA (TE), was loaded into a cuvette which was then inserted into the spectrophotometer chamber for measurement.
The polymerase chain reaction conditions were in accordance with CIMMYT laboratory protocols (2005), with minor modifications. The final concentrations of the PCR reagents that were used for amplification were; 40 ng template DNA, 0.25 µM forward and reverse primers, 1 unit Takara Ex Taq DNA polymerase (Separations, 150 µM each of dNTPs, 1X Taq buffer (20 mM Tris-HCI, pH 8.0,100 mM KCL, 0.1 mm EDTA, 1 Mm DTT, 0.5% Tween 20, 0.5% NP-40, 50% glycerol).
A touchdown PCR programme was used as described by Senior et al. (1998), with a few modifications. The initial cycle had a denaturation temperature of 94ºC for one minute. The second cycle had ten cycles, starting with denaturation at 94°C for 1 min, followed by annealing. One cycle was performed for every 1ºC decrease in annealing temperature from 65 to 55°C. Ten cycles were therefore performed at 10 different temperature settings. Extension was done at 72°C for 1 min 30 s. Temperature settings for the next 30 cycles were as follows; denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min 30 s. The final extension was at 72°C for 5 min, and the holding temperature was 4ºC. Each of the 27 SSR primers amplified DNA of each of the 45 inbred lines.
After amplification, PCR products were electrophoresed on a vertical gel system with 12% acrylamide solution (non-denaturing gels). A mixture of 6 µl of the PCR sample and 2 µl of O’Gene 6x orange loading dye (Thermo Scientific) were loaded into a 1.0 mm wide gel well. Products were separated by electrophoresis in a Bio-Rad Mini Protean Tetra System. The gels were run for 90 min at 120 volts. After electrophoresis, the gel was stained with 5 µl of ethidium bromide in 70 ml of distilled water at room temperature for 35 min. The bands of DNA were then visualised using a gel documentation system (Uvitec Cambridge, Alliance version 4.7). Allele sizes of the SSR bands were determined by comparing them with the internal O’Gene 100 bp molecular weight marker (CIMMYT, 2005).
Statistical analysis
For molecular analysis, each SSR primer was considered as a locus, and each band as an allele. Deoxyribonucleic acid banding patterns from SSR gels were converted to binary form, one indicated the presence of a specific allele and zero indicated its absence. The polymorphism information content (PIC) for each SSR primer was determined as described by Smith et al. (1997), using the following formula:
Where, 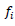 is the frequency of the ith allele. Gene diversity was calculated to quantify the genetic variation among the maize inbred lines. Allele frequency was calculated for each locus across the set of inbred lines using the Power Marker software version 3.25. The resulting unrooted tree was visualized using Mega version 5. The genetic distances between genotypes were computed using Roger’s (1972) genetic distances (RD). Cluster analysis was then carried out using the neighbour-joining tree (NJ) method.
is the frequency of the ith allele. Gene diversity was calculated to quantify the genetic variation among the maize inbred lines. Allele frequency was calculated for each locus across the set of inbred lines using the Power Marker software version 3.25. The resulting unrooted tree was visualized using Mega version 5. The genetic distances between genotypes were computed using Roger’s (1972) genetic distances (RD). Cluster analysis was then carried out using the neighbour-joining tree (NJ) method.
RESULTS
Polymorphism of SSR markers
The 27 SSR primers amplified a total of 121 bands among the 45 inbred lines, to give an average allele richness of 4.32 alleles per locus. The highest number of alleles (7) was identified for primer Phi127 and Phi 109275. The polymorphism information content (PIC) was from 0.20 for Phi 213984 to 0.67 for Phi109275, with a mean of 0.48. The gene diversity ranged from 0.23 to 0.71 while the average gene diversity was 0.53. Primer Phi 109275 showed the highest gene diversity (0.71) as shown in Table 2.
Genetic distances among the CIMMYT and QS inbred lines
The highest genetic distance was found between inbred lines CIM18 x QS 22 and between CIM19 x QS1 (0.46). The next highest genetic distance was 0.45, and it was found between inbred lines CIM1 x QS10; CIM15 x QS4; CIM16 x QS4; CIM15 x QS1; CIM16 x QS1; CIM19 x QS17; CIM19 x QS22; CIM19 x QS5; CIM3 x QS11 and CIM4 x QS19. Conversely, the lowest genetic distance was found between CIMMYT lines CIM12 x CIM13 (0.13). The overall average genetic distance was 0.31.
The highest genetic distances among the QS lines was 0.45. Cross combinations that exhibited this distance were QS9 x QS12 and QS6 x QS10. Other QS cross combinations with a moderate genetic distance of 0.43 were QS11 x QS21 while crosses QS16 x QS21; QS17 x QS21; QS19 x QS21 and QS2 and QS4 had a genetic distance of 0.41. It was observed that cross combinations within the QS cluster showed higher genetic distances (0.45) than cross combinations within the CIMMYT cluster (0.38).
Cluster analysis
The unrooted tree clearly revealed two distinct groups, with 22 and 23 inbred lines for clusters- 1 and 2 respectively (Figure 1). The CIMMYT inbred lines were grouped separately from QS inbred lines, with the exception of QS9 which branched off from CIM10. The major clusters were further divided into sub-clusters. Cluster 1 was further divided into four sub- clusters while cluster 2 was further divided into nine sub-clusters. Inbred lines did not cluster clearly according to heterotic groups or pedigree information provided, except for a few inbred lines such as QS3 and QS4; QS18 and QS19 which belonged to heterotic group F and H respectively.
DISCUSSION
Polymorphism of SSR markers
The average number of alleles per primer obtained in this study is less than those reported in previous SSR studies. However, the average allele number is in accordance with previous studies (Khoza, 2012) who also recorded average alleles of 4.96 per locus while investigating the genetic diversity of 60 maize inbred lines. The moderate allele richness and gene diversity in this study indicated a moderate genetic base. The average PIC value obtained (0.48), was higher than that reported by Legesse et al. (2007) of 0.33. The lowest PIC (0.20) was reported for primer Phi213984 which identified two alleles.
Genetic distance between CIMMYT and QS lines
There was greater diversity between CIMMYT and QS inbred lines than among lines within the major. This was evident from the genetic distances observed between the two groups of inbred lines. The highest genetic distance was recorded for CIM19 and QS1 which belonged to different clusters and heterotic groups, A and F respectively. This cross combination has the potential to produce superior hybrids. Hallauer and Miranda (1988) reported that the more parental lines are genetically distant the more likely the manifestation of heterosis. The lowest genetic distance was recorded for CIM12 and CIM13, which belonged to the same heterotic group, ‘B’ and were also grouped into the same sub-cluster. This indicated that CIM12 and CIM13 are less likely to develop high performing hybrids because they have almost similar genetic backgrounds.
Cross combinations with QS inbred lines showed moderate genetic diversity. This indicated that there was more diversity within the QS lines than there was among CIMMYT lines. The moderate genetic distance among QS lines may partly explain why it was possible to produce successful QPM hybrids that have been registered in South Africa so far. All inbred lines with a genetic distance ranging from 0.41 to 0.45 were taken from different sub-clusters within the QS cluster. The reason for low genetic distances for some maize inbred lines may be due to intensive breeding which aims to select germplasm suitable for similar agro- environments.
Cluster analysis
The QPM inbred lines were clustered into two major groups according to their source. CIMMYT-sourced inbred lines were distinctly separated from inbred lines sourced from Quality Seeds. The sub-clusters formed were expected because both CIMMYT and QS draw their inbred lines from different pools and populations (Warburton et al., 2005). QS9 was grouped with the CIMMYT inbred lines which have a tropical origin. According to the available information on QS9, its heterotic grouping is unknown but is assumed to have originated in the tropics (Gevers, Personal communication). Inbred lines CIM12 and CIM13 from CIMMYT were grouped together in the same sub-cluster because they share a common parent GQL5. The two lines probably inherited most of the genes from this common parent. Some of the lines were grouped according to heterotic groups while others were mixed.
According to Vivek et al. (2008), heterotic groups are subjective and are constantly evolving suggesting that heterotic groups such as ‘F’ F2834W may have been derived from the same population as H (Hickory King) which may explain the mix in groups in each cluster. The large number of sub-clusters found in the QS major cluster indicates a wider genetic diversity, as also shown by the several heterotic groups within that cluster. In comparison, the CIMMYT cluster showed only four sub-clusters and had two heterotic groups.
In conclusion, moderate genetic diversity was found in the selected QPM inbred lines based on 27 SSR markers. The clustering observed in this study was in agreement with some of the heterotic grouping. Quality protein maize inbred lines for hybridization can be selected based on the genetic distance information that was generated from this study.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The study was carried out with financial support from the Govan Mbeki Research and Development fund. The authors are grateful to Quality seeds cc and CIMMYT–Zimbabwe for providing the germplasm.
REFERENCES
|
CIMMYT (2005). Laboratory Protocols CIMMYT Applied Molecular Genetics laboratory. 3rd edition. Mexico D.F CIMMYT. |
|
|
Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK (2008). Marker assisted selection:an approach for precision plant breeding for the twenty first century. Phi. Trans. R. Soc. Lond. B. Biol. Sci. 363(1491):557-572. |
|
|
Department: Agriculture, Forestry, and Fisheries (DAFF) (2014). South African variety list as maintained by the registrar of plant improvement, pp. 35-37. |
|
|
Hallauer AR, Miranda JB (1988). Quantitative genetics in maize breeding 2nd edition. Iowa State University press, Ames Iowa USA. |
|
|
Khoza S (2012). Assessment of maize for genetic diversity, cultivar superiority, and combining ability.Master of Science dissertation. University of KwaZulu Natal, Pietermaritzburg, South Africa. |
|
|
Legesse BW, Myburg AA, Pixley K, Botha A (2007). Genetic diversity of maize inbred lines revealed by SSR markers. Heriditas. 144(1):10-17. |
|
|
Makumbi D, Betran JF, Banziger M, Ribaut JM (2011). Combining ability heterosis and genetic diversity in tropical maize (Zea Mays) under stress and non-stress conditions. Euphytica 180(2):143-162. |
|
|
Mondini L, Noorani A, Pagnotta MA (2009). Assessing plant genetic diversity by molecular tools. Diversity 1:19-35. |
|
|
Nuss ET, Tanumihardjo SA (2011). Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv. Nutr. 2(3):217-224. |
|
|
Prasanna BM, Vasal SK, Kassahun B, Singh NN (2001). Quality protein maize. Curr. Sci. 81(10):1308-1319. |
|
|
Pfunde CN (2013). Parent characterization of quality protein maize (Zea mays L.) and combining ability for tolerance to drought stress. Master of Science Dissertation, University of Fort Hare, Alice South Africa. |
|
|
Rogers JS (1972). Measures of genetic similarity and genetic distance. In: Studies in genetics VII. University of Texas Publication 7213, Austin, Texas. pp. 145-153. |
|
|
Senior MI, Murphy JP, Goodman MM, Stuber CW (1998). Utility of SSRs for determining genetic similarities and relationships in maize using an agarose gel system. Crop Sci. 38:1088-1098. |
|
|
Shin JH, Kwon SJ, Lee JK, Min HK, Kim NS (2006). Genetic diversity of maize kernel starch synthesis genes with snaps. Genome 49 (10):1287-1296. |
|
|
Smith JSC, Chin EC, Shu H, Smith OS, Wall SJ, Senior ML, Mitchell SE, Kresovich S, Ziegle J (1997). An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L.): comparisons with data from RFLPs and pedigrees. Theor. Appl. Genet. 95:163-173. |
|
|
Van Averberke W, Denison J, Mnkeni PNS (2011). Smallholder irrigation schemes in South Africa: A review of knowledge generated by the water research commission. Water SA 37:797-808. |
|
|
Van de Merwe BJ (1995). Cedara report on the current status of quality protein maize. KwaZulu Natal department of Agriculture. Cedara report No.N/A/95/35 |
|
|
Vivek BS, Krivanek AF, Palacio-Rojas N, Twumasi-Afiriye S, Diallo AO (2008). Breeding Quality Protein Maize: Protocols for developing QPM cultivars. Mexico, D.F: CIMMYT. |
|
|
Warburton ML, Ribaut JM, Franco J, Crossa J, Dubreuil P, Betran FJ (2005). Genetic characterisation of 218 elite CIMMYT maize inbred lines using RFLP markers. Euphytica 142:97-106. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0