ABSTRACT
The aim of this study was to determine how lipids, total soluble sugars and starch are degraded during the germination process of crambe seeds (FMS Brilhante Cultivar). The experiment consisted of collecting seeds during every day of the germination test (0, 1, 2, 3, 4, 5, 6 e 7 days) during which were also held the germination counts. After collection, the proportion of seeds and / or seedlings was designed for the determination of water content, and the other part was separated to perform biochemical analysis. The study adopted a completely randomized design with four replications and the means were compared by regression analysis. Crambe seed reserves showed degradation since the metabolic activation with increased germination in that lipid, soluble sugars, and starch are degraded.
Key words: Lipids, total soluble sugars, starch.
The crambe (Crambe abyssinica Hochst) belonging to the Brassicaceae family, originated from the Mediterranean region with occurrence in Ethiopia, and being recently introduced in Brazil as an alternative to forage and off-season crop production (Colodetti et al., 2012). The plant has a relatively short cycle of 90 to 120 days, requiring approximately 52 days between sowing and flowering, considerable tolerance to water stress, soil and saline irrigation water and also resistance to low temperatures (Pitol et al., 2010) and productivity of 1428,98 kg ha-1 (Brandão et al., 2013). The industrial use of crambe oil is suggested to be a raw material for biodiesel production because their seeds have oil content of up to 38% (Pitol et al., 2010) and features such as kinematic viscosity, density and acid value are suitable for biodiesel production (Silva et al., 2013).
The crambe oil extracted from the seed may be used as a lubricant in the manufacture of plastic films and in the drug composition. The crambe oil cannot be used for human consumption due to the presence of high erucic acid content, a long chain monounsaturated fatty acid, which causes damage to the heart when present in the human body (Colodetti et al., 2012).
In addition to the purely industrial point of view, it should be considered that the yield obtained in the industries is potentially dependent on the quality and yield of plants. Thus, the germination of this kind becomes important to any industrial process which is intrinsically associated with the mode degradation of its reserves. The main substance stored in crambe seeds are lipids, but carbohydrates and proteins are also found into smaller proportions (Oliva et al., 2012).
During germination, reservations are hydrolyzed and mobilized to embryo growth (Kucera et al., 2005). Lipids and carbohydrates are used as an energy source (Pritchard et al., 2002) and protein to provide amino acids for the formation of new tissues (Ramakrishna, 2007).
Plants need to convert the more lipids stored in a mobile form of carbon, often as sucrose, because they are not able to carry fats cotyledon to other tissues during development of the seedlings (Taiz and Zeiger, 2013). According to Graham (2008) oilseeds metabolize the triacylglycerols stored, converting them to sucrose following germination. The conversion of sucrose to lipid in oil seed germination is initiated by starting with the hydrolysis of stored triglycerides lipids as free fatty acids, and subsequent oxidation of these fatty acids to produce acetyl-CoA. Fatty acids are oxidized in glioxissomo, and acetyl-CoA is metabolized in glioxissomo and cytoplasm to produce succinate, which is transported to mitochondria and converted to fumarate and then malate, and cytosolic malate is converted to glucose via gluconeogenesis and then as sucrose (Taiz and Zeiger, 2013).
Considering the importance of the degradation of reserves during the germination of seeds, the aim of this study was to determine how lipids, total soluble sugars and starch are degraded during the germination process of crambe seeds.
Installation, experimental design and data analysis
The seeds were purchased from the Mato Grosso do Sul Foundation. The experiment was conducted in the Germination, Seed Dormancy and Plant Physiology Laboratory II, of the Department of Botany, Biosciences Institute, UNESP – Botucatu / SP. The experiment consists of seeds collection during every day of the germination test (0, 1, 2, 3, 4, 5, 6 and 7 days) during which the germination counts were also held. Four replications of 50 seeds were placed for each treatment. After collection, the proportion of seeds and/or seedlings was designed for the determination of water content, and the other part was separated to perform biochemical analyzes.
A completely randomized design was adopted with four replications and the means were compared by regression analysis (p≤0.05).
Seed moisture content
The water content was determined by the oven method at 105 ± 3°C for 24 h, using three replicates of 4.5± 0.5 g. The results were expressed on a wet basis (Brasil, 2009).
Germination
The germination test was conducted with four replications of 50 seeds for each treatment. The seeds were placed on blotting paper, moistened in water for 2.5 times the mass of dry substrate, packed in transparent plastic boxes (11 x 11 x 3.5 cm). They were then placed on germination B.O.D. in alternating temperature and photoperiod (light - 30°C for 8 h and dark - 20°C for 16 h).
Total soluble sugars
The extraction of total soluble sugars was carried out according to the methodology of Garcia et al. (2006) with minor modifications. The seeds were pulverized with a pestle and mortar in N2 liquid. To obtain the alcoholic extract, 100 mg of the pulverized seeds were homogenized in 1 mL of 80% ethanol (v/v) and incubated for 15 min at 80°C. The homogenate was centrifuged at 12,000 g for 15 min at room temperature. At the end of centrifugation, the supernatant was removed and reserved. This procedure was performed three times for the complete removal of total soluble sugars, combining the supernatants from the three extractions at the end. Then, the final volume was adjusted to 3 ml with deionized water resulting in the alcoholic extract. These extract was stored in separate microtubes at -20°C until determination. The pellets were stored at -20°C for further starch extraction.
For the determination of total soluble sugars, the methodologies used were taken from Morris (1948) and Yemm and Willis (1954). The anthrone reagent was prepared dissolving 0.1 g of anthrone in 45 mL of sulfuric acid 95% (v/v). The reaction mixture consisted of 50 µL of alcoholic extract + 950 μL deionized water (final volume 1000 μL), kept in an ice bath, and 2000 μL of cold anthrone solution was added. The reaction mixture was incubated for 3 min at 100°C. After cooling, the total sugar content was determined by taking the absorbance at 620 nm using glucose as a standard and was expressed as mg per mg of dry weight.
Starch
For extraction of starch, the study followed the the method described by Clegg (1956) with minor modifications. The pellet, derived from the alcoholic extract, was homogenized in 500 µL of deionized water, in an ice bath. To the homogenate was added 650 μL of 52% perchloric acid (v/v) and kept in an ice bath for 15 min (shaken every 5 min). Then, 2000µL of deionized water was added and centrifuged at 12,000 g for 15 min at 4°C.
At the end of centrifugation, the supernatant was removed and reserved. This procedure was performed again, however the mixture containing pellet + deionized water + 52% perchloric acid (v/v) was kept in an ice bath for 30 min (shaken every 5 min) and centrifuged at 12,000 g for 15 min at 4°C. The two supernatants were combined at the end. These extract was stored in separate microtubes at -20°C until determination of starch content. The determination of starch content was performed like the total sugar determination.
Total lipid
The quantification of total lipids was performed according to the method of Manirakiza et al. (2001) and Ambalkar et al. (2011). To obtain the extract, about 1.0 to 2.0 grams of sample soaked in liquid nitrogen was weighed. In these samples, 100 ml of hexane was added, arranged in flat-bottom flasks and placed in fatty material extract in 3 cycles of 8 h. Subsequently, the material was filter while hot, and brought to a rotary evaporator for separation of solvent and lipids. Lipids were removed from the flasks with the aid of a Pasteur pipette, transferred to glass jars with lid, and subsequently weighed.
The results of germination of crambe seed, obtained during the 7-day evaluation and the degradation reservations are presented in tables. It can be seen that seed germination process started on the third day of evaluation.
The data relating to lipid content in the seeds set to a decreasing quadratic regression equation, indicating that there has been rapid consumption of reserves during the germination period from the first day after the metabolic activation (Figure 1).
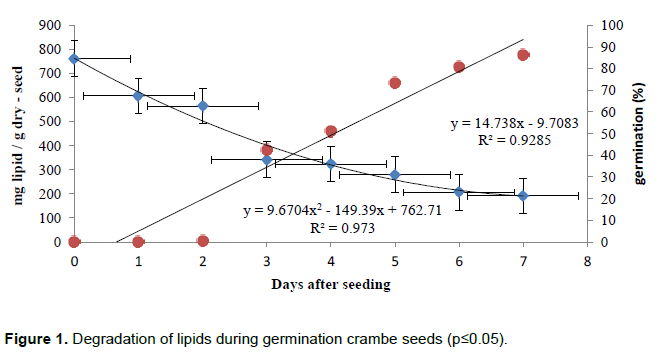
In Cucumis sativus L. the degradation of lipids started on the 2nd day after germination, leaving only 3% of total initial six days. This rapid degradation begins with the emergence of the radicle and ends with the complete expansion of the cotyledons (Matsui et al., 1999). However, according Suda & Giorgini (2000) this pattern is unusual when compared to other oil seeds, in which the lipid content remains unchanged during the initial period of germination, diminishing. It was observed in Arabidopsis thaliana (L.) that the degradation of lipid reserves is inhibited in the presence of soluble sugars such as glucose and sucrose, and in general stemmed starch metabolism (To et al., 2002).
The total soluble sugar seeds also exhibit marked reduction during the initial stage of germination, during imbibition (Day 1), indicating their use in breathing, with subsequent stabilization of cotyledons between 3 and 6 days (Figure 2) and decrease in the seventh day. Borges et al. (2002) and Buckeridge and Dietrich (1996) verified the consumption of sucrose and raffinose during germination and Sesbania marginata, Platymiscium pubescens, respectively, considering the first two reservations of soluble sugars to be used. The data relating to starch content in the seed set to a downward quadratic regression equation indicates the intake and mobilization of reserves during the germination period (Figure 3). According to Magalhaes et al. (2010), starch provides glucose to be used both as air for breath, to generate electricity, and to compose physical structures for embryo growth during germination phase.
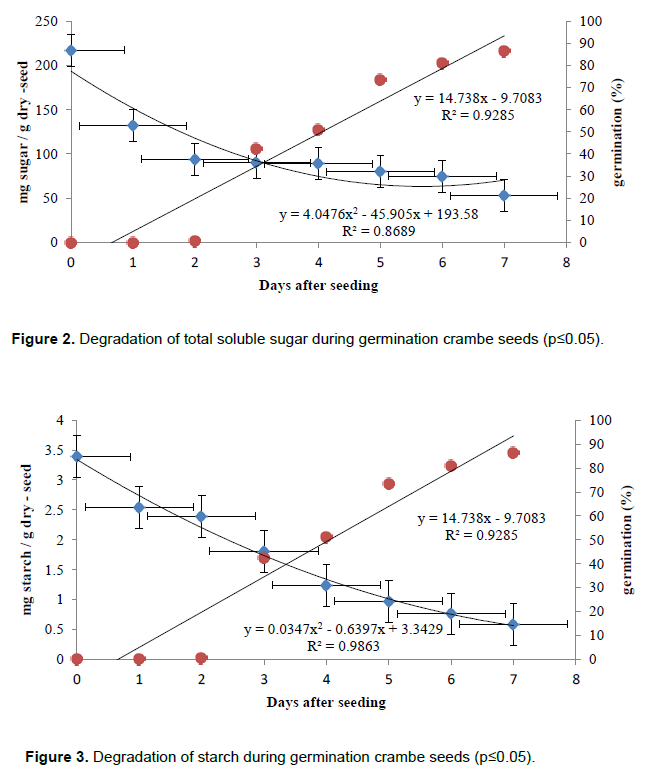
In summary, crambe seed germination lipids, soluble sugars and starch are degraded quickly providing energy for the development of the embryo during germination, which is completed at 7 days after metabolic activation, when observing the emission of the primary root. Thus, while lipid reserves are considered slow in degradation, crambe seed process starts from the moment the seeds are placed in contact with water and the metabolism is activated.
Crambe seed reserves had degradation since the metabolic activation with increased germination in that lipid, soluble sugars and starch are degraded.
The authors have not declared any conflict of interest.
The São Paulo State University CAPES and CNPq are thanked for their financial support.
REFERENCES
|
Ambalkar VU, Sapkal VS, Talib M, Khandelwal SA (2011). Soxhlet extraction of Neem seed (Azadirachta indica A. Juss) using hexane as a solvent. Int. J. Chem. Anal. Sci. 2:2.
|
|
|
|
Borges EEL, Borges RCG, Soares CPB, Perez SCJGA (2002). Crescimento e mobilização de carboidrato em embrião de sementes de fedegoso (Senna macranthera Irwin et Barneby) durante a germinação. Cerne. Lavras 8(1):69-76.
|
|
|
|
|
Brandão FJB, Silva ARB, Silva MAP, Sperotto FCS (2013). Desempenho operacional e produtividade agrícola do crambe nos preparos convencional e reduzido de solo. Enciclopédia Biosfera. Centro Científico Conhecer - Goiânia 9:17.
|
|
|
|
|
Brasil (2009). Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. 399p.
|
|
|
|
|
Buckeridge MS, Dietrich SMC (1996). Mobilization of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth (Leguminosae Faboideae). Plant Sci. Amsterdan 117:33-43.
Crossref
|
|
|
|
|
Clegg KM (1956). The application of anthrone reagente to the estimation of starch in cereals. J. Sci. Food Agric. 7(1):40-44.
Crossref
|
|
|
|
|
Colodetti TV, Martins LD, Rodrigues WN, Brinate SVB, Tomaz MA (2012). Crambe: Aspectos Gerais da Produção Agrícola. Enciclopédia Biosfera, Centro Cientifico Conhecer, Goiânia 8(14):258-269.
|
|
|
|
|
Garcia IS, Souza A, Barbedo CJ, Dietrich SMCE, Figueiredo-Ribeiro RCL (2006). Changes in soluble carbohydrates during storage of Caesalpinia echinata Lam. (Brazilwood) seeds, an endangered leguminous tree from the brazilian atlantic forest. Braz. J. Biol. 66(2B):739-745.
Crossref
|
|
|
|
|
Graham IA (2008). Seed storage oil mobilization. Ann. Rev. Plant Biol. 59:115-42.
Crossref
|
|
|
|
|
Kucera B, Cohn MA, Leubner-Metzger G (2005). Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. Wallingford 15:281-307.
Crossref
|
|
|
|
|
Magalhaes SR, Borges EEL, Berger APA (2010). Mobilização de reservas no eixo embrionário e nos cotilédones de sementes de Schizolobium parahyba (vell.) S. f. blake durante a germinação. Ciência Florestal Santa Maria 20(4):589-595.
Crossref
|
|
|
|
|
Manirakiza P, Covaci A, Schepens P (2001). Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh & Dyer extraction methods. J. Food Composit. Anal. 14(1):93-100.
Crossref
|
|
|
|
|
Matsui K, Hijiya K, Tabuchi Y, Kajiwara T (1999). Cucumber Cotyledon Lipoxygenase during Postgerminative Growth. Its Expression and Action on Lipid Bodies. Plant Physiol. 119:1279-1287.
Crossref
|
|
|
|
|
Morris DL (1948). Quantitative determination of carbohydrates with dreywood's anthrone reagent. Science 107(2775):254-255.
Crossref
|
|
|
|
|
Oliva ACE, Biaggioni MAM, Cavariani C (2012). Efeito imediato do método de secagem na qualidade de sementes de crambe. Energ. Agric. Botucatu. 27(3):16-30.
Crossref
|
|
|
|
|
Pitol C, Broch DL, Roscoe R (2010). Tecnologia e produção: crambe 2010. Fundação MS, Maracajú. Fundação MS. 60p.
|
|
|
|
|
Pritchard SL, Charlton WL, Baker A, Graham IA (2002). Germination and storage reserve mobilization are regulated independently in Arabidopsis. Plant J. 31(5):639-647.
Crossref
|
|
|
|
|
Ramakrishna V (2007). Mobilization of albumins and globulins during germination of Indian bean (Dolichos lablab L. var. lignosus) seeds. Acta Bot. Croat. 66(2):135-142.
|
|
|
|
|
Silva MAP, Biaggioni MAM, Sperotto FCS, Bezerra PHS, Brandão FJB (2013). Qualidade do oleo bruto de grãos de crambe (Crambe abyssinica Hochst) sob diferentes métodos de secagem. Energ. Agric. Botucatu. 28(3):193-199.
|
|
|
|
|
Taiz L, Zeiger E (2013). Fisiologia Vegetal. 5. Ed. Porto Alegre. Artmed.
|
|
|
|
|
To JPC, Reiter W, Gibson SI (2002). Mobilization of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biol. 2:4-15.
Crossref
|
|
|
|
|
Yemm EW, Willis AJ (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57(3):508-514.
Crossref
|
|