ABSTRACT
Due to the need for proper water quality for human consumption, some studies have developed new treatment techniques. Some have used a natural coagulant based on Moringa oleifera, which has the capacity to clarify turbidity water and has anti-microbial properties. This paper aimed at developing a new technique to apply the coagulant. This technique consists of putting the M. oleifera powder into pouches. Five different materials were analyzed for the concentration of protein released and the increased turbidity in the water: four different types of nonwoven fabric and one commercial coffee filter paper. It was made some tests of dissolution: during 24 h, the pouches were put in distilled and deionized water and then it was mixed in low velocity in the Jar Test equipment. Through these tests, it was found that the five pouch materials released protein into the water even in the first 60 min of contact. It gave 7.7 mg L-1 min-1 of dispersion rate and maintained turbidity values lower than the ones observed using the liquid coagulant. From the result, it’s possible to conclude that those five types of pouches can be used in water treatment, because all them release similar concentration of protein that was observed when it was used the liquid coagulant.
Key words: Moringa oleifera, pouch, protein concentration.
Access to water with good quality for human consumption is the subject of many research studies, because of its implications for the health of a population. That is one of the reasons of the fundamental importance of the consumption of contaminants free water. Thus, subjects such as water quality and its reuse are increasingly being discussed and studied.
An option to improve the efficiency of physical treatment systems is the use of coagulants to aid the shaping of clusters which are easily removed in the process of sedimentation or filtration. However, the use of synthetic coagulants can represent a high financial value and can leave unwanted residue in the treated water depending on the conditions of the essay. Because of this reason, the use of plant-based coagulants has become a better option for the clarification of turbid waters.
One of the natural coagulants that are already studied is the one that is produced from the powder of Moringa oleifera seeds. Composed of approximately 40% protein, (Gallão, 2006), M. oleifera seeds are quite used in the clarification of turbid waters in some regions of the planet, such as Sudan and other African countries, Northeast Brazilian, among others (Borba, 2001). This protein is responsible by the processes of coagulation and flocculation of colloids in natural water that present color and turbidity. According to Ndabigengesere et al. (1995), it is a hydro soluble cationic protein, which has molecular mass between 12 and 14 kDa. However, Broin et al. (2002) and Ghebremichael et al. (2005) saw that when this protein is in aqueous solution and it is responsible by the coagulation process, it has molecular mass between 6.0 and 6.5 kDa. Ndabigengesere et al. (1995), Ndabigengesere and Narasiah (1998) and Amagloh and Benang (2009) saw that the use of M. oleifera in water treatment could result in a similar efficiency when it is compared with aluminum sulfate.
The use of M. oleifera as a coagulant in rural communities can show advantages like the generation of biodegradable sludge (Bergamasco et al., 2009). Depends of the characteristic of the water that will be treated, this sludge can be applied as fertilizer (Ndabigengesere and Narasiah, 1998). Furthermore, the volume of sludge that the use of the M. oleifera had created is lower than the one created by the aluminum sulfate (Bergamasco et al., 2009). Ndabigengesere and Narasiah (1998) verified that there were not significant changes in pH parameter because of the use of M. oleifera. For many tested dosages, this parameter remained around 7.6. Valverde et al. (2013) also verified that M. oleifera can operate over a wide pH range and it does not have a significant change in the treated water pH. Babu and Chaudhuri (2005), Beltrán -Heredia and Sánchez-Martín (2009) obtained a big reduction in turbidity when they used a M. oleifera seeds aqueous suspension as auxiliary on the direct filtration. However, Babu and Chaudhuri (2005) verified the fast colmatation of the filter. Using the coagulant based on the M. oleifera seeds as an auxiliary in the slow sand filtration process, Franco et al. (2010) found that its use can result in a smaller duration in the career of filtration. Pritchard et al. (2010), in addition to the conventional method of dosing the coagulant, tested an alternative way of performing this dosage: they confined the M. oleifera seed powder into pouches made of muslin.
However, based on the results obtained, the authors found that an optimization of the method is required.Based on the recommendations of Pritchard et al. (2010) and also considering that not all materials of the M. oleifera seed operates as a coagulant, the objective of this study was develop a new method for the application of the coagulant solution, reducing the addition of possible residues that are not interesting to the water to be treated.
The research was developed in the Hydraulics and Irrigation Laboratory and in the Sanitation Laboratory of the Faculty of Agricultural Engineering at the University of Campinas (FEAGRI/UNICAMP). The M. oleifera seeds were collected in the Experimental Field of FEAGRI/UNICAMP, dried in a heater at 65°C for 24 h and stored in an incubator with temperature and humidity control.
At the time of preparation of the pouches, the seeds were peeled and processed in manual grinder. All the five types of pouches received 2 g of powder obtained after grinding. The characterization of this powder was made according to Henderson and Perry (1976), and it was established that such powder was not considered a uniform product, being composed of medium and fine grain with an average diameter of 0.388 mm. All pouches had approximately 31 cm2 of contact area. After the powder was added into the pouches, they were sealed and attached to one side of the jar.
Five types of materials were evaluated for the manufacture of the pouches: four non-woven fabrics weighting 272 g m-2 (NW 272), 352 g m-2 (NW 352), 117 g m-2 (NW 117),42 g m-2 (NW 42) and one Melitta® filter paper, produced in Guaíba/RS/BR, weighting 54 g m-2 (FP). The non-woven fabrics were made with polymers. The characteristics of each of these materials are described in Table 1. The thickness was determined using a pachymeter and the relation between the materials weight and the samples area determined the grammage. The permeation and the resistance to the airflow were determined by the Gurley method (NBR NM:ISSO 5636) developed by Packaging Technology Center/ Institute of Food Technology in Campinas/SP. The porosity (or pore percentage) and the pore size was determined using image analysis: the images were obtained by sweep electrical microscopy and processed using the computational program (Rasband and ImageJ, 2014).
Figure 1 represents each of the five types of pouches used. Besides the pouches, we also evaluated the application of liquid coagulant (LC) based on M. oleifera seeds. For the production of the aqueous solution, M. oleifera seeds were manual peeled and then they were processed in a manual grinder and were sieved in a sieve with 0.84 mm of opening. It was added that powder to distilled and deionized water aiming to obtain 20 g L-1 of concentration. After the homogenization of the powder in water using a magnetic agitator for 3 min, a sieve with 0.125 mm of opening passed through the resultant suspension. At least, it was added 100 mL of this solution to one of the jars.

The assays were conducted in Jar-Test equipment. It were added 2 L of distilled and deionized water in each jar in the equipment. In the first jar, it was added an aqueous solution based on 2% M. oleifera seeds, under the hypothesis of representing the most critical situation of turbidity and protein. In each of the other five jars, it was positioned one of five types of pouches, as shown in Figure 2. The assays were carried out for 24 h. It were collected samples at the instants: 0, 5, 10, 15, 20, 25, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, 480, 540, 600, 660, 720, 780, 840, 960, 1080, 1200, 1320 and 1440 min. Throughout the period, the Jar-Test was kept under slow agitation, with 20 rotations per min (RPM) as described by Pritchard et al. (2010) in order to maintain the homogeneity of the medium. For the proposed evaluation, turbidity analyses were made by nephelometry and concentration of protein by the method of Lowry (1951), adapted by Madrona (2010). The method of Lowry consists in the reduction of folin reagent when it is in the presence of a protein that was previously treated with copper.
This reaction can result in a “new” component that gives color to the solution with 550 nm of maximum absorption. The standard curve was obtained using Bovine serum albumin (BSA).
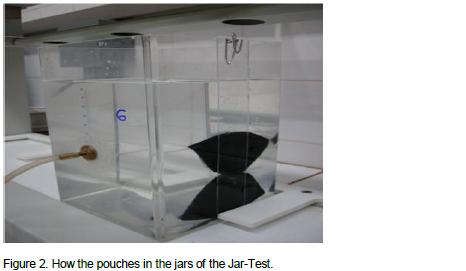
The average turbidity of the distilled and deionized water after the addition of the pouches and liquid coagulant (LC) are represented in Figure 3. With the use of the five pouches, it was found that after 600 min of dispersion, increased turbidity values were observed. There were increased turbidity values with the use of the liquid coagulant since the first min of the dispersion. In the treatment with LC, flakes were probably formed and deposited, fact that can justify the gradual reduction of turbidity over time.
The pouch made with filter paper (FP) was the one that caused the lowest turbidity increase. At the end of the 24 h, the increased turbidity arising from the use of the pouches NW 272, NW 352, NW 117 and NW 41 was respectively, 534, 590, 441 and 296% higher than that observed with the use of filter paper. These percentages are relating to turbidity increase by the pouches NW 272, NW 352, NW 117 and NW 41, when they were compared to the filter paper (the lowest value of turbidity that was observed) after 24 h of contact. However, for all treatments carried out with the use of pouch, it was observed maximum turbidity values below that were verified with the use of liquid coagulant (LC). Comparing the greater turbidity value observed with the use of liquid coagulant (0 min) with such value for the other pouches (1440 min), it was found that the increase in turbidity from the liquid coagulant was 1553, 161, 140, 205 and 317% higher than that observed with the use of the pouches Filter Paper, NW272, NW352, NW117 and NW 41, respectively. In this case, the higher value of turbidity was verified in each treatment were compared, based on the turbidity of original water as reference.
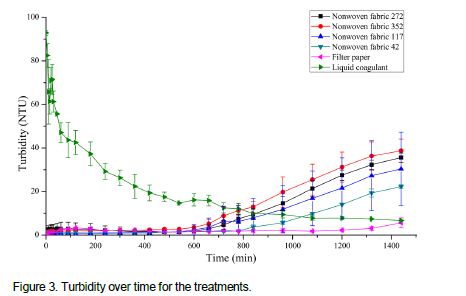
There is the possibility that the reason of the raise in the turbidity during the essay was the solubilization of some substances that were contained in M. oleifera seeds. Figure 4 demonstrates that, for the five treatments with pouch, the concentration of protein over time presented very similar values, with the greatest increase in concentration at the beginning of the assays and a tendency for stabilization after 480 min. During the preparation of the LC, the M. oleifera seed powder was added to the distilled and deionized water, with the agitation of such a mixture. This way, with the use of the LC, it was observed high concentrations of protein at the beginning of the assay. The average dispersal rate (DP) is the rate of change of protein concentration (ΔCi) for a given period of time (Δt) and is given by the equation:

As shown in Figure 5, the pouches that provided protein for the medium at a higher speed were those made with NW 272 and NW 352. At 5 min, for those pouches, Vdisp was 7.7 mg L-1 min-1 and 7.5 mg L-1 min-1, respectively. For the other treatments, the maximum dispersion rate did not exceed 5.5 mg L-1 min-1. However, in such treatments, the dispersion rate exceeded 1.0 mg L-1 min-1 up to 60 min, while, when using the pouches NW 272 and NW 352 at 60 min, the dispersal rate was less than 1.0 mg L-1 min-1. For the five treatments, after 60 min, it were observed values lower than 1.0 mg L-1 min-1 for the dispersal rate, with a predominance of values below 0.1 mg L-1 min-1. In the direct application of LC, it was verified that at 30 and 45 min of dispersion there are spikes in the concentration of protein, with Vdisp equal to 3.0 mg L-1 min-1 and 2.0 mg L-1 min-1, respectively. In the other times, Vdisp is close to or less than 0.0 mg L-1 min-1.
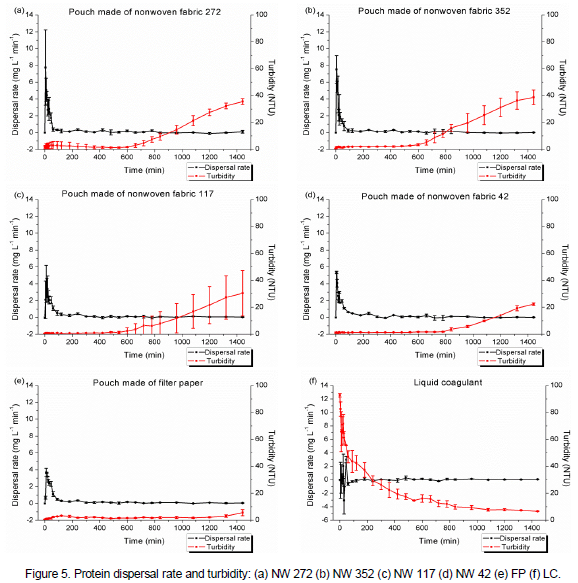
The five types of pouches used provide considerable concentrations of protein in the first minutes of dispersion, which enables their use for the treatment of water, since the protein is the agent responsible for the process of coagulation and flocculation (Madrona et al., 2009). Although the use of the pouch made of filter paper has resulted in a smaller increment of turbidity in the distilled and deionized water, with the use of the other pouches, increased turbidity values were only observed from the times in which the dispersal rate is considerably low, as shown in Figure 5.
Considering the criteria evaluated in this study, we conclude that the five types of pouches can be used. As in all treatments the protein concentration reached values similar to those observed with the use of liquid coagulant in a dispersal period in which the turbidity still had low values. Pritchard et al. (2010) used muslin pouches containing powder of M. oleifera seeds in water treatment and they obtained 85% of reduction in turbidity. It shows that this technology has potential to reduce the turbidity in water treatment.
The authors have not declared any conflict of interest.
The authors thank São Paulo Research Foundation (FAPESP) for the scholarship granted for the research (FAPESP process No. 2010/09395-0). They also thank Espaço da Escrita – Coordenadoria Geral da Universidade - UNICAMP for the translation of the manuscript into English.
REFERENCES
|
Amagloh FK, Benang A (2009). Effectiveness of Moringa oleifera seed as coagulant for water purification. Afr. J. Agric. Res. 4(1):119-123. |
|
|
|
Babu R, Chaudhuri M (2005). Home water treatment by direct filtration with natural coagulant. J. Water Health 3(1):27-30. |
|
|
Beltrán-Heredia J, Sánchez-Martín J (2009). Improvement of water treatment pilot plant with Moringa oleifera extract as flocculant agent. Environ. Technol. 30(6):525-534.
Crossref |
|
|
|
Bergamasco R, Moraes LCK, Cardoso KC, Vieira MAS, Madrona GS, Klen MRF (2009). Diagramas de coagulação utilizando Moringa oleifera lam e o sulfato de alumínio, visando remoção de cor e turbidez da água. In: Congresso Brasileiro de Engenharia Sanitária e Ambiental, Recife/PE /BR. |
|
|
|
Borba LR (2001). Viabilidade do uso da Moringa oleifera lam no tratamento simplificado de água para pequenas comunidades. João Pessoa, Paraíba: Universidade Federal da Paraíba. (Dissertação de Mestrado). |
|
|
Broin M, Santaella C, Cuine S, Kokou K, Peltier G, Joët T (2002). Flocculent activity of a recombination protein from Moringa oleifera Lam. Seeds. Appl. Microbiol. Biotechnol. 60:114-119.
Crossref |
|
|
|
Franco M (2010). Uso de coagulante extraído de sementes de Moringa oleifera como auxiliar no tratamento de água por filtração em múltiplas etapas. Campinas, São Paulo: Universidade Estadual de Campinas (Dissertação de Mestrado). |
|
|
|
Gallão MI, Damasceno LF, Brito ES (2006). Avaliação química e estrutural da semente de Moringa. Revista Ciência Agronômica, 37(1):106-109. |
|
|
Ghebremichael KA, Gunaratna KR, Henriksson H, Brumer H, Dalhammar G (2005). A simple purification and activity assay of the coagulant protein form Moringa oleifera seed. Water Res. 39:2338-2344.
Crossref |
|
|
|
Henderson SM, Perry ME (1976). Agricultural Process Engineering. The AVI Publ. Com., Capítulo 6: Size Reduction. |
|
|
|
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem.193:265-275. |
|
|
|
Madrona GS, Serpelloni GB, Nishi L, Bergamasco R, Vieira MAS, Araújo AA, Abreu Filho BA (2009). Avaliação do peso molecular de diferentes extratos obtidos da semente de Moringa oleifera lam e sua aplicação para obtenção de água potável. In: Silva GF, Bergamasco R, Miranda CSA, Serafini MR (2009). Potencialidades da Moringa Oleifera Lam. UFS:São Cristovão, 1:233-250. |
|
|
|
Madrona GS (2010). Estudo da extração/purificação do composto ativo da semente da Moringa oleifera Lam e sua utilização no tratamento de água de abastecimento. Maringa, Paraná: Universidade Estadual de Maringá. (Tese de Doutorado) . |
|
|
Ndabigenegesere A, Narasiah KS, Talbot BG (1995). Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 29 (2):703-710.
Crossref |
|
|
Ndabigenegesere A, Narasiah KS (1998), Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 32(3):781-791.
Crossref |
|
|
Pritchard M, Craven T, Mkandawire T, Edmondson AS, O'Neill JG (2010). A comparison between Moringa oleifera and chemical coagulants in the purification os drinking water – An alternative sustainable solution for developing countries. Phys. Chem. Earth, 35(13-14):798-805.
Crossref |
|
|
|
Rasband WS, ImageJ US (2014). National Institutes of Health, Bethesda, Maryland, USA. |
|
|
|
Valverde KC, Moraes LCK, Bongiovani FPC, Bergamasco R (2013). Coagulation diagram using the Moringa oleifera Lam and the aluminium sulfate, aiming the removal of color and turbidity of water. Acta Scientarium. Technol. 5(3):485-489. |






