Full Length Research Paper
ABSTRACT
Melon (Cucumis melo L.) is a cucurbitaceous of great appreciation worldwide. The intensive cultivation of melons has favored the increase of plant health problems in several producing regions. Among these problems, the root-knot nematode (Meloidogyne spp.) stands out. The development of genetically resistant cultivars is consolidated as an effective strategy for the management of these pathogens, it is then essential to screen cultivars and accessions for later identification of genetic sources of resistance. This study aimed to evaluates the reaction of melon genotypes to Meloidogyne enterolobii. The essay was conducted at the Sector of Vegetable Crops and Aromatic-Medicinal Plants of UNESP-FCAV Jaboticabal Campus, in greenhouse, from March to June, 2015. It was evaluated 18 melon genotypes, two commercial cultivars 'Fantasy' and 'Louis', and as susceptibility control, the tomato 'Santa Cruz Kada'. A completely randomized design was adopted, with 21 treatments and 7 repetitions. The total number of eggs and juveniles in the roots (TNEJ) and the reproduction factor (RF) were obtained in order to determine the reaction of each genotype evaluated. The accessions PI 414723, AC 29, and PI 124112 are resistant to M. enterolobii and are therefore promising for breeding programs.
Key words: Cucumis melo, reproduction factor, plant breeding, root-knot nematode.
INTRODUCTION
The melon (Cucumis melo L.) is a cucurbitaceous of great appreciation worldwide, which uses it in many ways, for the preparation of juices, fruit salads and for fresh consumption. A diverse offering of fruits of this species is a differential, and these vary in shape, flavor, flesh color, aroma, among other aspects. The cropping systems are also diverse, depending basically on the type of melon intended to be commercialized. The noble melons, on account of its high commercial value, are commonly grown in greenhouses (Peil, 2003; Ito et al.,2014). In turn, the yellow melons are open-field grown, in large extension areas.
The intensive cultivation of cucurbits has promoted the development of nematodes that results in significant losses in highly infest crops (Gallati et al., 2015). For cucurbits, the most common species are Meloidogyne incognita, Meloidogyne javanica, and Meloidogyne arenaria (Pinheiro and Amaro, 2010).
Recently, another species, Meloidogyne enterolobii, although it occurs less frequently in relation to M. incognita and M. javanica, is becoming increasingly important, since many reported melon genotypes as resistant to major root-knot nematodes show no resistance to this species (Brito et al., 2007; Cetintas et al., 2007; Cantu et al., 2009; Kiewnick et al., 2009; Eppo, 2011; Melo et al., 2011; Westerich et al., 2011; Castagnone-Sereno, 2012; Singh et al., 2013).
Some authors believe that the cultivation of plants resistant to other species of nematodes can cause, eventually, a selection pressure in favor of M. enterolobii, and can take it to the status of primary economic importance. In some countries, M. enterolobii is classified as a quarantine pest (Castagnone-Sereno, 2012; Elling, 2013).
The nematode belonging to M. enterolobii species was first described by Yang and Eisenback (1983) from the roots of Enterolobium contortisiliquum (Vell.) Morong. Rammah and Hirschmann (1988) classified the same nematode as Meloidogyne mayaguensis, from eggplant roots (Solanum melongena L.). Later, with the use of more sophisticated methodologies, it was established that M. mayaguensis is actually a young form of M. enterolobii, being these two species synonyms and the second nomenclature should be adopted (Xu et al., 2004; Karssen et al., 2012).
Symptoms of M. enterolobii are characterized by leaf yellowing, reduced plant growth and root galls (Eppo, 2011), and interactions may also occur with other pathogens (Pinheiro and Amaro, 2010). Although there are few reports (Pinheiro et al., 2014; Bitencourt and Silva, 2010) in the literature regarding M. enterolobii in melon, the occurrence of this pathogen in other cucurbits is indicative that this nematode can potentially cause economic damage to melon crops. The identification of sources of resistance to M. enterolobii is therefore of fundamental importance for breeding programs. The use of genetically resistant plants is the most sustainable method to control Meloidogyne spp., being a challenge the search for sources of resistance (Molinari, 2011). Alternatively, however, in a short-term, the use of resistant rootstocks would be feasible, as practiced for other crops (Louws et al., 2010; Thies et al., 2012; Galatti et al., 2013; Guan et al., 2014). Nevertheless, this practice would have greater applicability in noble melons cultivated in greenhouses, because of the high commercial value-added. This work aimed at select melon genotypes resistant to M. enterolobii, in order to use the sources of resistance to start breeding programs.
MATERIALS AND METHODS
The experiment was conducted at the Sector of Vegetable Crops and Aromatic-Medicinal Plants of UNESP- FCAV Jaboticabal Campus, in greenhouse, from March to June 2015. A randomized complete block design was adopted, with 21 treatments and seven replicates. It was considered as repetition a plant inoculated with M. enterolobii per pot. As control of susceptibility, the tomato 'Santa Cruz Kada' was used.
The melon genotypes used were Vendrantais, PI-140471, PI-432398, PI 420150, PI 5322830, PMR-5, PI-157082, WMR-29, Charentais Fom 1, PI-420145, C160, CNPH 01- 930, Nantais Oblong, AC 29, PMR-45, PMR-6, PI 414723, PI 124112, used as differentiators of powdery mildew races and gummy stem blight and the commercial cultivars Louis and Fantasy. The inoculum were obtained from a subpopulation of M. enterolobii, extracted from guava 'Paluma' roots, coming from Taquaritinga, Sao Paulo State, Brazil . The species were identified at the Nematology Laboratory of UNESP-FCAV Jaboticabal Campus, using a photonic microscope TNB-40T-PL. The identification was based on the morphological characters of the perineal pattern, prepared as Taylor and Netscher (1974), on the morphology of the males lip region (Eisenback et al., 1981) and on the esterase isozyme phenotype, obtained by the technique Esbenshade and Triantaphyllou (1990), using a traditional vertical electrophoresis system Mini Protean II from BIO-RAD.
The subpopulation was previously multiplied in potted eggplant (Solanum melongena L.) 'Anápolis', in greenhouse. In order to obtain the initial inoculum, after 90 days of inoculation, eggplant plants were removed from pots and the roots were washed and pounded in a blender with 0.5% sodium hypochlorite (Hussey and Barker, 1973). The estimation of eggs and juveniles population presents in suspension was carried out with the aid of Peters counting chamber, using a photonic microscope, with subsequent adjustment of the concentration at 1000 eggs and second stage juveniles/mL and inoculation of 5 ml of this suspension per seedling. The melon and tomato seedlings were produced in 128 cells polystyrene trays using the commercial substrate Bioplant®, in greenhouse equipped with sprinkler irrigation system. It was seeded two seeds per cell, with subsequent thinning.
When the seedlings were 25 days-old, the transplant was held. It was used two-liters plastic pots. The substrate was composed of a mixture of soil, sand and cattle manure, at the ratio 1:1:1. This mixture was previously autoclaved (120°C, 1 atm, 1 h). At transplanting, it was also held up the inoculation of the suspension containing eggs and second stage juveniles of M. enterolobii. The nematode species identity was confirmed at the Nematology Laboratory of UNESP-FCAV Jaboticabal Campus. For this, it was used the perineal pattern as Taylor and Netscher (1974), and morphology of male lip region, according to Eisenback et al. (1981). All inoculated plants were analyzed at 60 days after transplanting and inoculating the nematodes.
All roots were gently washed in a bowl with water, in order to remove the excess of soil. It was then processed for the extraction of nematodes eggs and juveniles, according to Hussey and Barker (1973). The final population of each suspension was derived from individually processed root systems and estimated by counting eggs and juveniles with the aid of Peters counting chamber, using a photonic microscopy. This population was used for determining the reproduction factor (RF), whereas plants with FR<1 were considered as resistant, and those with FR≥1, susceptible to the nematode, according to Oostenbrink (1966).
The data were transformed to 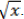 Analyses were performed using the statistical software Genes (Cruz, 2013), and averages were grouped by the Scott and Knott test (p <0.01). Phenotypic, genotypic and environmental variances, as well as the ratio CVg/CVe were estimated.
Analyses were performed using the statistical software Genes (Cruz, 2013), and averages were grouped by the Scott and Knott test (p <0.01). Phenotypic, genotypic and environmental variances, as well as the ratio CVg/CVe were estimated.
RESULTS
There were differences (p<0.01) among genotypes for the total number of eggs and juveniles (TNEJ) and reproduction factor (RF) when inoculated with M. enterolobii. Therefore, this analysis assumes that chance produces only small deviations, and the major differences are generated by real causes. For this reason, we used this type of analysis) (Table 1). The environmental variation coefficients for total number of eggs and juveniles and reproduction factor were 30.08 and 30.21, respectively.
The variables TNEJ and RF showed values of CVg/CVe of 1.42 and 1.15, respectively, indicating that a selection of resistant genotypes through phenotypic traits, would be effective. M. enterolobii inoculation was efficient, since there was multiplication of the nematode in tomato 'Santa Cruz Kada', which presented averages FR>1 and TNEJ larger than what was inoculated (Table 2). For TNEJ, it was established three groups according to average grouped by the Scott and Knott test. The susceptible control (tomato 'Santa Cruz Kada') had the highest average (43598) and access ‘PI 124112', the lowest average (1594).
For RF, it was formed four groups of averages by the Scort and Knot test, with the tomato 'Santa Cruz Kada' presenting the highest value and 'PI 124112’ the lowest, averaging 8.7 and 0.3, respectively. Based on the reproduction factor (RF), as Oostenbrink (1966), the accessions that presented RF<1 were considered resistant to M. enterolobii, namely: ‘PI 414723’, ‘AC 29’, and ‘PI 124112'. Similarly, other materials were considered susceptible for presenting RF≥1. All materials classified as resistant were not immune to M. enterolobii, having, as the other tested materials, hosted the nematode. Comparing TNEJ, seven materials did not differ statistically from the three genotypes considered as resistant, namely: ‘Charentais Fom 1’, 'PI-420145', 'C160', 'CNPH-01930', ‘Nantais Oblong ', ‘PMR-45’, and ‘PMR-6’.
DISCUSSION
The mean square analysis of variance is significant, which may be indicative of the existence of variability between genotypes. The higher coefficient of variation can be explained by the greater environment influence on the characteristic in question, since the variable response results from interaction between two biological factors (nematodes x plants). There are reports of several works in which were also obtained high coefficients of variation, being characteristic of this type of essay (Wilcken et al., 2005; Freitas et al., 2008).
It is observed that, for both evaluated characteristics, most of the phenotypic variance was attributed to genetic effects (Table 1). This result expresses the reliability of the obtained results, by indicating that the responses had low environment influence and are highly determined by genetic effects. Based on the favorable results in some genotypes, for resistance to M. enterolobii, it is noted that there is the possibility of selection for resistance in subsequent generations. One way to increase the efficiency of breeding programs would make selection based on the average of progenies selection (Carvalho Filho et al., 2011).
When the reason CVg/CVe presents greater or equal to one values indicates that the gains from selection are favorable for a certain characteristic, due to the positive difference of genetic variation compared to the environmental variation (Vencovsky and Barriga, 1992).
Silva et al. (2002) points out that since the melon breeding involves various interest features, it is interesting to study the genetic, phenotypic and environmental correlations. Thus, as the work related to resistance to this pathogen progress, it has the ability to verify these correlations featuring more effectively the resistance of melon genotypes to M. enterolobii.
In view of the reported resistance to M. enterolobii in three genotypes, it is possible to perform selection for that characteristic, since the genotypes described in this work are also being used in other lines of research, which enables the incorporation of more interest features to the genotype within the breeding programs.
Although some studies have reported resistance in yellow melon to M. incognita and M. javanica (Bitencourt and Silva, 2010; Marques et al., 2012; Galatti et al., 2013; Ito et al., 2014; Lopez-Gomez and Verdejo-Lucas, 2014), there are few studies that show resistance, or even susceptibility for melons in relation to M. enterolobii. The results are promising in view of the scarcity of studies related to this nematode in melon. There are several reports of cultivars of various species resistant to most root-knot nematodes, but susceptible to M. enterolobii. Cantu et al. (2009) evaluated eight tomato rootstocks (Solanum lycopersicum L.) informed as resistant to M. incognita, M. javanica and M. arenaria, and observed that the rootstocks behaved as susceptible to M. enterolobii. A work on the parasitism of this nematode held in cowpea (Vigna unguiculata L.) 'IPA-9', 'IPA 206' and the tomato cultivars ‘Santa Cruz’ and ‘Viradoro’ demonstrated susceptibility reactions (Guimarães et al., 2003).
In species of Capsicum spp., only C. frutescens was considered resistant M. enterolobii (Oliveira, 2007). In lettuce, variations of reaction to some root-knot nematode species are also reported (Gomes et al., 2000; Maluf et al., 2002; Carvalho Filho et al., 2008; Silva et al., 2008; Bitencourt and Silva, 2010). According to Yang and Eisenback (1983), melon is a good host for M. enterolobii, making it difficult to manage this crop in fields infested by this pathogen. The evaluation of resistant materials, such as those obtained in this study, allows the identification of promising materials for the rootstocks or to the transfer of resistance genes in subsequent works. According to Peil (2003) grafting has been used in Cucurbitaceae vegetable crops (watermelon, melon, cucumber and pumpkin) in Brazil, which have features that enables grafting.
In this study, the genotypes (Vendrantais, PI 140471, PI 432398, PI 420150, PI 5322830, PMR-5, PI 157082, WMR-29, Charentais Fom 1, PI 420145, C160, CNPH 01- 930, Nantais Oblong, PMR-45, PMR- 6, along with the cultivars 'Louis' and 'Fantasy') increased the initial population, being classified as susceptible, and three genotypes (PI 414723, AC 29, and PI 124112) did not increased the initial population, and are therefore resistant. Thus, it was found resistant genotypes to this nematode, however none is immune. Immunity is characterized by interactions between the host and pathogen, being the host the plants exposed to the pathogen, which produces defense substances (Williamson, 1999; Williamson and Kumar, 2006). The resistance of melon genotypes can be further assessed in heritage studies, as the resistance genes are usually specific, contemplating few species of Meloidogyne, at where the resistance can be conferred by one, a few or many genes (Williamson and Roberts, 2009). From the results obtained in this work, further studies on melon breeding programs with PI 414723, AC 29, and PI 124112, resistant to M. enterolobii, is possible.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors acknowledge the support of the Universidade Estadual Paulista (UNESP) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
REFERENCES
|
Bitencourt NV, Silva GS (2010). Reprodução de Meloidogyne enterolobii em Olerícolas. Nematology. Bras. 34:181-183. |
|
|
Brito JA, Stanley JD, Kaur R, Cetintas R, Di Vito M, Thies JA (2007). Effects of the Mi-1, N and Tabasco genes on infection and reproduction of Meloidogyne mayaguensis on tomato and pepper genotypes. J. Nematol. 39:327-332. |
|
|
Cantu RR, Wilcken SRS, Rosa JMO, Goto R (2009). Reação de porta-enxertos comerciais de tomateiro a Meloidogyne mayaguensis. Sum. Phytopathol. 35:216-218. |
|
|
Carvalho Filho JLS, Gomes LAA, Westerich JN, Maluf WR, Campos VC, Ferreira S (2008). Inheritance of resistance of 'Salinas 88' lettuce to the root-knot nematode Meloidogyne incognita (Kofoid & White) Chitwood. Rev. Bras. Agrocienc. 14:279-289. |
|
|
Carvalho Filho JLS, Gomes LAA, Silva RR, Ferreira S, Carvalho RRC, Maluf WR (2011). Parâmetros populacionais e correlação entre características da resistência a nematoides de galhas em alface. Rev. Bras. Cienc. Agrar. 1:46-51. |
|
|
Castagnone-Sereno P (2012). Meloidogyne enterolobii (= M. mayaguensis): profile of an emerging, highly pathogenic, root-knot nematode species. Nematology 14:133-138. |
|
|
Cetintas R, Kaur R, Brito JA, Mendes ML, Nyczepir AP, Dickson DW (2007). Pathogenicity and reproductive potential of Meloidogyne mayaguensis and M. floridensis compared with three common Meloidogyne spp. Nematropica 37:21-31. |
|
|
Cruz CD (2013). Genes - a software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 35:271-276. |
|
|
Eisenback JB, Hirschmann H, Sasser JN, Trintaphyllou AC (1981). A guide to the four most common species of root-knot nematodes (Meloidogyne spp.) with a pictorial key. Departments of Plant Pathology and Genetics, North Carolina State University, Raleigh, USA. |
|
|
Elling AA (2013). Major emerging problems with minor Meloidogyne species. Phytopathology 103:1092-1102. |
|
|
Esbenshade PR, Triantaphyllou AC (1990). Isozyme phenotipes for the identification of Meloidogyne species. J. Nematol. 22:10-15. |
|
|
Freitas JAD, Santos GCD, Souza VS, Azevedo SMD (2008). Resistência de clones de batata-doce, Ipomoea batatas L., aos nematóides causadores de galhas. Acta Sci. Agron. 23:1257-1261. |
|
|
Galatti FS, Franco AJ, Ito LA, Charlo HCO, Gaion LA, Braz LT (2013). Rootstocks resistant to Meloidogyne incognita and compability of grafting in net melon. Rev. Ceres 60:432-436. |
|
|
Gomes LAA, Maluf WR, Campos VP (2000). Inheritance of the resistant reaction of the lettuce cultivar 'Grand Rapids' to the southern root-knot nematode Meloidogyne incognita (Kofoid & White) Chitwood. Euphytica 114:37-46. |
|
|
Guan W, Zhao X, Dickson DW, Mendes ML, Thies J (2014). Root-knot nematode resistance, yield, and fruit quality of specialty melons grafted onto Cucumis metulifer. HortScience 49:1046-1051. |
|
|
Guimarães LMP, Moura RM, Pedrosa EMR (2003). Parasitismo de Meloidogyne mayaguensis em diferentes espécies botânicas. Nematol. Bras. 27:139-145. INIST : 21029, 35400011681476.0030 |
|
|
Hussey RS, Barker KR (1973). A comparasion of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 57:1025-1028. |
|
|
Ito LA, Gaion LA, Galatti FS, Braz LT, Santos JM (2014). Resistência de porta-enxertos de cucurbitáceas a nematoides e compatibilidade da enxertia em melão. Hortic. Bras. 32:297-302. |
|
|
Karssen G, Liao JL, Kan Z, Van Heese E, Den Nijs L (2012). On the species status of the root-knot nematode Meloidogyne mayaguensis Rammah & Hirschmann, 1988. ZooKeys 181:67-77. |
|
|
Kiewnick S, Dessimoz M, Franck L (2009). Effects of the Mi-1 and the N root-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. J. Nematol. 41:134-139. |
|
|
López-Gómez M, Verdejo-Lucas S (2014). Penetration and reproduction of root-knot nematodes on cucurbit species. Eur. J. Plant Pathol. 139:863-871. |
|
|
Louws FJ, Rivard CL, Kubota C (2010). Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci. Hortic. 127:127-146. |
|
|
Maluf WR, Azevedo SM, Gomes LAA, Oliveira ACB (2002). Inheritance of resistance to the root-knot nematode Meloidogyne javanica in lettuce. Gen. Mol. Res. 1:64-71. |
|
|
Marques MLS, Pimentel JP, Tavares OCH, Veiga CFM, Berbara RLL (2012). Hospedabilidade de diferentes espécies de plantas a Meloidogyne enterolobii no Estado do Rio de Janeiro. Nematropica 42:304-313. |
|
|
Melo OD, Maluf WR, Gonçalves RJS, Gonçalves Neto AC, Gomes LAA, Carvalho RC (2011). Triagem de genótipos de hortaliças para resistência a Meloidogyne enterolobii. Pesqui. Agropecu. Bras. 46:829-835. |
|
|
Molinari S (2011). Natural genetic and induced plant resistance, as a control strategy to plant-parasitic nematodes alternative to pesticides. Plant Cell Rep. 30:311-323. |
|
|
Oliveira CD (2007). Enxertia de plantas de pimentão em Capsicum spp. no manejo de nematóides de galha. Thesis (PhD in Agronomy) 134 f. |
|
|
Oostenbrink M (1966). Major characteristics of the relation between nematode and plants. Meded. Landbouwhogeschool 66:3-46. |
|
|
Peil RM (2003). A enxertia na produção de mudas de hortaliças. Cienc. Rural 336:1169-1177. |
|
|
Pinheiro JB, Amaro GB (2010). Ocorrência e controle de nematoides nas principais espécies cultivadas de cucurbitáceas. Circular Técnica, Embrapa Hortaliças. |
|
|
Pinheiro JB, da Silva RC, Pereira RB, de Carvalho ADF, Suinaga FA (2014). Boletim de Pesquisa e Desenvolvimento 103. |
|
|
Rammah A, Hirschmann H (1988). Meloidogyne mayaguensis n.sp. (Meloidogynidae), a root-knot nematode from Puerto Rico. J. Nematol. 20:58-69. |
|
|
Silva RA, Bezerra NF, Nunes GHS, Negreiros MZ (2002). Estimação de parâmetros genéticos e correlações em famílias de meios-irmãos de melões Orange Red Flesh e HTC 01. Caatinga 15:43-48. |
|
|
Silva RR, Gomes LAA, Monteiro AB, Maluf WR, Carvalho Filho JLS, Massaroto JA (2008). Linhagens de alface-crespa para o verão resistentes ao Meloidogyne javanica e ao vírus mosaico-da-alface. Pesqui. Agropecu. Bras. 43:1349-1356. |
|
|
Singh SK, Hodda M, Ash GJ (2013). Plant- parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. Bulletin OEPP/EPPO Bulletin 43:334-374. |
|
|
Taylor AL, Netscher C (1974). An improved technique for preparing perineal patterns of Meloidogyne spp. Nematology 20:268-269. |
|
|
Thies JA, Ariss JJ, Hassell RL, Levi A (2012). Response of cucurbit rootstock for grafted melon to root-knot nematodes. J. Nematol. 44:494. |
|
|
Vencovsky R, Barriga P (1992). Genética biométrica no fitomelhoramento. Genet. Mol. Biol. 1992:486. |
|
|
Westerich JN, Rosa JMO, Wilcken SRS (2011). Estudo comparativo da biologia de Meloidogyne enterolobii (= M. mayaguensis) e Meloidogyne javanica em tomateiros com gene Mi. Sum. Phytopathol. 37:35-41. |
|
|
Wilcken A, Silvia RS, Garcia MJM (2005). Resistência de alface do tipo americana a Meloidogyne incognita raça 2. Nematol. Bras. 2:267-271. |
|
|
Williamson VM (1999). Plant nematode resistance genes. Curr. Opin. Plant Biol. 2:327-331. |
|
|
Williamson VM, Kumar A (2006). Nematode resistance in plants: the battle underground. Trends Genet. 22:396-403. |
|
|
Williamson VM, Roberts PA (2009). Mechanisms and genetics of resistance. In: Perry RN, Moens M, Starr JL (Ed.). Root-knot nematodes. CAB INTERNATIONAL, Wallingford, UK. pp. 301-325. |
|
|
Xu J, Liu P, Meng Q, Long H (2004). Characterization of Meloidogyne species from China using isozyme phenotypes and amplified mitochondrial DNA restriction fragment length polymorphism. Eur. J. Plant Pathol. 110:309-315. |
|
|
Yang B, Eisenback JD (1983). Meloidogyne enterolobii n.sp. (Meloidogynidae), a root-knot nematode parasitizing pacara earpot tree in China. J. Nematol. 15:381-391. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0