Date palm (Phoenix dactylifera L.) cultivation is one of the most economically-important activities in the arid zones of the Middle East and North Africa (El Hadrami and Al-Khayri, 2012). One of the major problems in date palm cultivation that prevents rapid crop improvement is the lack of an adequate method of asexual propagation.
Traditionally, date palm is propagated vegetatively by offshoots produced by desirable trees. The low rate of propagation (1-20 offshoots/ tree, depending on the variety) has limited the multiplication of healthy plants (Tisserat, 1982). To overcome the propagation problems and to maintain the germplasm, the in vitro micropropagation. Large-scale plant production through cell tissue and embryo cultures using bioreactors is promising for industrial plant propagation. Bioreactors are usually described in a biochemical context as self-contained, sterile environments which capitalize on liquid nutrient or liquid/air in?ow and out?ow systems, designed for intensive culture and according maximal opportunity for monitoring and control over micro environmental conditions such as agitation, aeration, temperature, dissolved oxygen and pH (Paek et al., 2005). The application of liquid cultures for micropropagation in bioreactors using the embryogenic and organogenic regeneration pathways is becoming a more efficient alternative system for scale-up and automation in vitro (Ziv, 1995; Tahardi et al., 2003; Ducos et al., 2007). However, somatic embryogenesis appears to be the most appropriate for an automated system in liquid medium (Etienne et al., 2006). The cultivation in liquid media using a temporary immersion system with different frequencies of immersion was reported to improve plant quality and multiplication rates of banana and coffee (Alvard et al., 1993; Teisson and Alvard, 1995). In addition, Othmani et al. (2009) demonstrated the applicability of embryogenic suspension culture for high output of date palm somatic embryos using temporary immersion bioreactor (TIB) system. The objective of the present study is to develop the use of other possible explants (leaf segments) as the sources of in vitro culture for date palm cv. Quntar, as well as development of somatic embryos in culture system with temporary tissue immersions "bioreactor", and optimize the protocol for indirect shoot.
Plant material
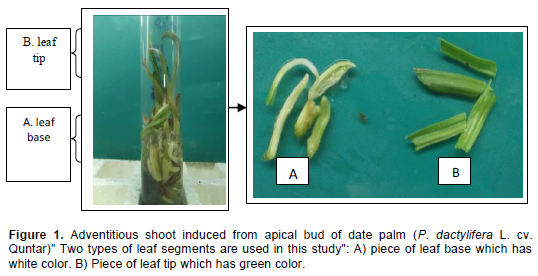
The experiment was carried out at the Plant Tissue Culture Laboratory, Basra University, Iraq. Two types of leaf segments (piece of leaf base which has white color, and piece of leaf tip which has green color) (Figure 1A and B). These pieces were taken from plantlets of date palm (P. dactylifera L. cv. Quntar) produced in in vitro culture. After, the larger cut of leaf was trimming into segments at 1 cm length. To induce callus, these leaf segments were horizontally cultured directly in Murashige and Skoog, 1962).’MS medium’, with additional 100 mgl-1 glutamine, 5 mgl-1thiamine HCl, 1 mgl-1 biotin, 40 mgl-1adenine sulfate, 30 gl-1 sucrose, 2.0 gl-1 activated charcoal. Also the media were supplemented with different concentrations of 2,4-D (0.0, 1.0, 2.5 and 5 mgl-1) combined with 1 mgl-1 BA. The pH of medium was adjusted to 5.8 before adding 7 gl-1 agar. The media were sterilised by autoclaving at 1.1 kg cm-2 (121°C) for 20 min. All the cultures were incubated at 27±2°C in darkness. The frequency of explant producing callus and fresh weights (gm) of callus were recorded for callusing explants after 6 weeks. The primary calluses were chopped and transferred on the same medium, after two subcultures with six weeks interval, embryogenic calluses were used for the installation of embryogenic suspension cultures and indirect shoot organogenesis.
PLANT DEVELOPMENTAL PATHWAYS FROM EMBRYOGENIG CALLUS
Somatic embryogenesis
Effect of culture media composition in culture system with temporary tissue immersions "bioreactor" on Somatic embryo production
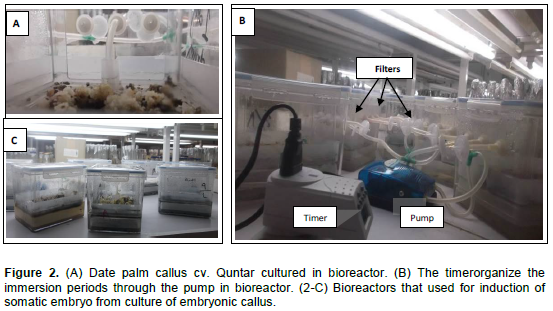
Embryogenic callus pieces (500 mg), ]developed from piece of leaf base which has white color of date palm (cv. Quntar)[ were culturing in bioreactor with 400 ml liquid medium (Figure 2A). The culture medium contained MS basal salts, 170 mgl-1 KH2PO4, 10 mgl-1 thiamine-HCl, 200 mgl-1 glutamine, 30 gl-1 sucrose and 0.500 gl-1 AC. This medium supplemented with different concentrations of 2,4- D (0.0, 0.5, 1.0 and 2.0 mgl-1). A temporary immersion system (TIS) was conducted using bioreactor. The callus in bioreactors remained in their culture containers at 5 weeks. The fresh mass of callus was evaluated at every subculture. The immersion duration for the latter was 3 min every 8 h. Once the best combination of culture medium was determined, a second experiment was carried out, to evaluate the effect of the immersion period on somatic embryo development as follows: 1 min every 4 h, 5 min every 8 h and 10 min every 12 h (Figure 2B). In both two experiments, three bioreactors containers were used (Figure 2C). The suspension cultures were kept under 16 h light photoperiod (30 µmol m-2 s-1) at 27 ± 2°C. For both experiments, the total number of somatic embryos, was determined after 45 days from culture.
Germination of the somatic embryos
Matured somatic embryos of date palm were transferred to MS medium enriched with 0.1 mg l-1 NAA. The MS basal medium was added with 30 g/L sucrose, pH 5.7 and solidified with 6 gl-1 Agar for shoot and root induction. The cultures were incubated in the culture room at 27 ± 2°C with 16 h light and 8 h dark.
Organogenic pathway
Indirect shoot organogenesis from leaf derived callus
For shoot regeneration, embryonic callus obtained from the above experiments was transferred to MS medium supplemented with various of cytokinins (BA alone, 2iP alone or in combination with auxin NAA, for each experiment a minimum of 12 replicates. The data was recorded after 12 weeks from culturing as following: The percentage of response of callus mass on shoot regeneration; number of regeneration shoots /explant. For improvement of shoot regeneration, the medium was optimized by testing the effect of different concentrations of gelling agents (6, 7, and 8 gl-1 agar or 2, 3, and 4 gl-1 Gelrite). Cultures were maintained at 27 ± 2°C in a growth chamber with a 16 h photo-period under standard cool white fluorescent tubes (35 µmol s-1 m-2) and 60 to 70% relative humidity.
Elongation and rooting
Regeneration shoots were transferred to the MS medium supplemented with 0.5 mgl-1 GA3 + 1.0 mg1-1 NAA for elongation and rooting.
Acclimatization
All the produced plantlets from indirect regeneration via somatic embryogenesis and shoot organogenesis were acclimatized. The plantlets were gently removed from the vessels, washed initially to remove adhered gelling agent and traces of the medium to avoid contamination. Then, the plantlets were washed with distilled water and treated with fungicide (Benlet 500 mgl-1) for 20 min and transferred to plastic pots containing autoclaved a mixture of peat moss and perlite (2:1) (AL-Mayahi, 2014). The plants were covered with glass bottles to maintain humidity. The plants were initially irrigated with quarter-strength inorganic salts of MS medium for 2 week followed by tap water. Potted plantlets were grown in culture room (25±2°C, 55±5% RH, under 16 h of photoperiod with a light intensity of 40 μmol m-2 s-1) for 45 days. The glass bottles were gradually removed upon emergence of new leaves and acclimatized plantlets were transferred to the greenhouse.
Histological studies
Histological examinations during bud formation were carried out using a freezing microtome. Microtome slide preparation and observation were made following the methods as described by Sarker and Awal (1999).
Experimental design and statistical analysis
A complete randomized design was employed in all of the experiments. Analysis of variance was used to show statistical differences between treatments using the L.S.D. at 5% (Snedecor and Cochran, 1989).
Formation of primary and embryogenic callus
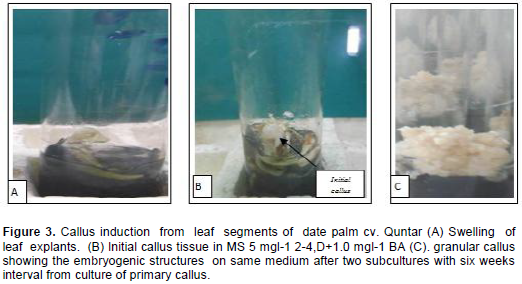
The first evidence of date palm callus induction process was the swollen of leaf segment when it cultured on MS medium supplemented with different concentrations of 2-4,D+ BA after 3 weeks of incubation (Figure 3A). Maximum response callusing response from the [piece of leaf base (white)], reached 53.34% in the medium MS + 5 mgl-1 2-4,D+1.0 mgl-1 BA after 5 weeks of incubation (Figure 3B). These same media gave the highest fresh weight of callus reached 1.239 g. (Table 1). The weight in this treatment which was statistically significant compared with the other treatments media.. Also, variations existed between two types of leaves segments in their ability to form callus. The piece of leaf base produced considerably more callus than piece of leaf tip which did not show any response for callus formation (Table 1). When the globular and compact primary callus were cultivated on the same medium. This callus gave rise to friable granular callus composed of embryogenic cells after two subcultures with six weeks interval (Figure 3C).
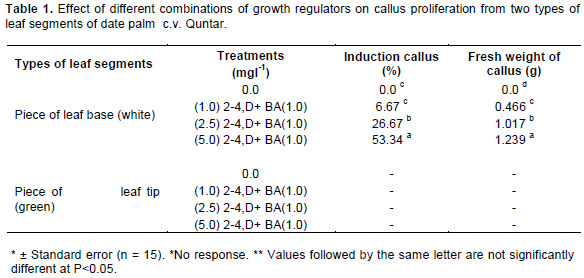
According to the results shown in Table 1, leaves cultured on MS medium supplemented with 5 mgl-1 2-4,D+1.0 mgl-1 BA gave better callus formation. The ratio of auxin: cytokinin was critical for inducing callus at a high frequency. Using certain hormonal balance in which the percentage of the auxins is much bigger than the percentage of the cytokenines, results in unbinding and breaking up the cohesive tissue structure of the basic explant, and also in cell division, which led to important swelling in the size of the basic explants, followed by the appearance of the callus tissue. Of all the auxins or auxin-like plant growth regulators, 2,4-D has proven extremely useful, being used in 57.1% of successful embryogenic cultures (Al-Khalifah and Shanavaskhan, 2012). Callus induction has been reported from almost all types of tissues of date palm on various media formulations, which usually contained a combination of an auxin and a cytokinin (Taha et al., 2002; Al-Mayahi 2013). The type of tissue reactions were influenced by many factors but mostly by medium composition and the level of tissue differentiation and lignification (Abahmane, 2013). This good response of explants for callus formation may be due to the efficient selection of cells in an explants (Al-Mallah and Hassan, 1994). Coupled with compatibility between the endogenous hormonal levels and the addition of growth regulator to the induction medium. This may also suggest that levels of endogenous hormones or their sensitivity might vary between organs. These results are similar to those reported by Sane et al. (2012) who confirmed that the aptitude for primary callogenesis appeared to be strongly dependent on the explant nature, the growth regulators used and the genotype.
Effect of culture media composition in culture system with temporary tissue immersions "bioreactor" on Somatic embryo regeneration
The effect of the four liquid media supplemented with different concentrations of 2,4-D in culture system with temporary tissue immersions on somatic embryo formation from embryogenic callus derived from piece of leaf base (white) (Table (2). The highest number of somatic embryos was obtained in media containing 1.0 mgl-1 2,4-D After 45 days of culture reached 172 embryos/ bioreactor (500 mg embryonic callus) (Figure 4A). This treatment was significantly different other treatments after 60 days from culture in system with temporary tissue immersions, (Table 2). Followed liquid media supplemented with 2.0 mgl-1 2,4-D gave rise to only 78 somatic embryos. The germination rates of somatic embryos were 82% after transfer to MS medium enriched with 0.1 mg l-1NAA. (Figure 4C) Also, Figure 4D and E showed the plants proliferation (shoots and roots) in same medium after 60 days from embryos culture.
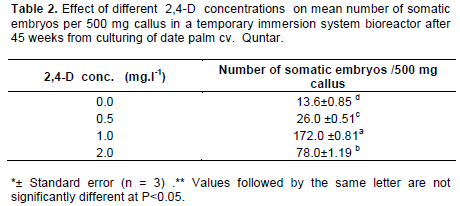
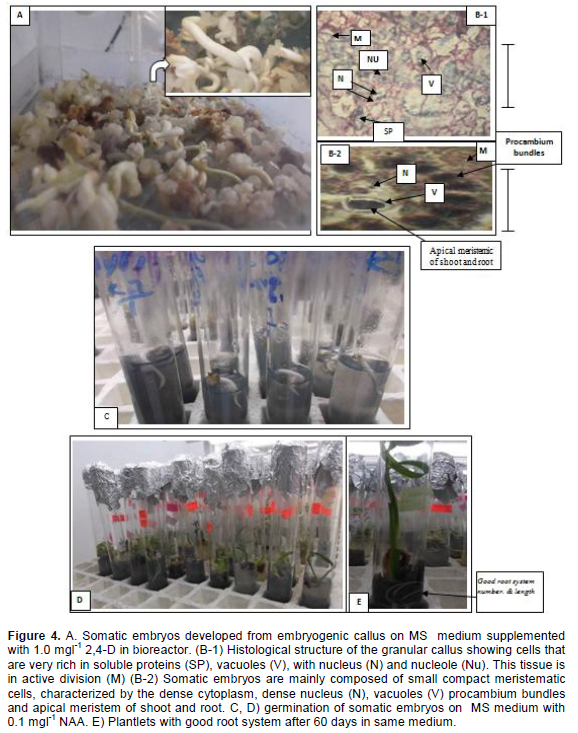
A histological study showed embryogenic cells are mainly composed of nucleus (N) nucleole (Nu) after 2-3 weeks of culture of the globular callus in the bioreactor. These cells of tissue were in active division (M) showed very small vacuoles and a large quantity of soluble proteins in the cytoplasm stained blue (Figure 4B-1). The somatic embryos started their development of meristematic centers after 6 weeks of culture. Somatic embryos are mainly composed of small compact meristematic cells, characterized by the dense cytoplasm, dense nucleus (N), vacuoles (V), procambium bundles and apical meristem for shoot and root.
Induction of somatic embryos from embryogenic callus cultures of were established in the present study using bioreactor was successfully obtained. Using 2-4,D was important to unbind and break up the components of the tissue structure to separate cell or tissue groups (Margara, 1984). It is well established that 2,4-D plays a role in the induction of somatic embryogenesis which is presumably mediated by a signal cascade triggered by this exogenous auxin (Zuo et al., 2002). Somatic embryogenesis is considered the most efficient regeneration process for date palm micropropagation (Fki et al., 2003). It is reported to be a quick and efficient method for large scale propagation of date palm and could also be highly useful for breeding programs (El Hadrami et al., 1998). The great increment in the number of somatic embryos in culture system with temporary tissue immersions "bioreactor" include better contact between the plant biomass and the medium, no restrictions of gas exchange, the control of the composition of both the medium and the gaseous atmosphere.
The improvement in somatic embryo formation and maturation in culture system with temporary tissue immersions "bioreactor" may be attributed to the increased oxygen diffusion to the liquid medium and eventually to the developing embryogenic cells because of gradually higher agitation as well as air supply in bioreactor. The bioreactor transfers oxygen from gaseousto the liquid stage, makes oxygen available to cultivating tissues or cells and promotes in vitro biomass growth (Sajc et al., 2000; Sane et al., 2006). The optimum presence of several gases like O2 and CO2 are very critical for somatic embryo as the requirement varies at different stages of embryo growth (Rosnow et al., 2011). Similar observations have earlier been reported for carrot (Jay et al., 1992). Also, this confirms previous findings reported that temporary immersion culture systems have improved the development of somatic embryos in Musa spp and C. arabica F1 hybrid (Caturra x E531), and woody plants (Escalant et al., 1994; Barry-Etienne et al., 2002; Paek and Chakrabarty, 2003). Ibraheem et al. (2013) reported that the liquid culture system (suspension cultures and RITA® bioreactors), is a promising technique for rapid mass propagation of date palm, somatic embryo production in liquid media is about ten times greater than that on solid media. Ducos et al. (2007), who found that the temporary immersion system has positive effects on micropropagation as somatic embryogenesis for coffee. This system is very efficient, because it reduces the toxicity of phenols emitted by in vitro cultivated tissue (Fki et al., 2011). The present study also was demonstrated that the use bioreactor was fostered embryo initiation (Data not shown). This result is in accordance with Ibraheem et al. (2013) who found that the liquid medium accelerated the formation of somatic embryos. These embryos were formed within 3-6 weeks after inoculation in the liquid media. While, they were formed in 12 to 18 weeks on solid medium.
During the transition from embryogenic status to somatic embryogenic status, cells have to dedifferentiate and activate their cell division cycle. Dividing small cells had an embryogenic appearance, with a protein-rich cytoplasm, small vacuoles and a large nucleus. Dividing small cells had an embryogenic appearance, with a protein-rich cytoplasm, small vacuoles and a large nucleus as described in by Sane et al. (2006), the absence of accumulation of reserves during the maturation of the somatic embryos, and finally the development of these embryos into rooted vitro plants (Figure 4B-2).
Effect of the immersion duration on production of somatic embryo
The Figure 5 showed that effect of the immersion period on somatic embryo production of date palm cv. Quntar. The immersion period in the bioreactors significantly affected the formation of somatic embryos. The highest number of somatic embryos, were obtained using an immersion period of 1 min every 4 h. Increasing the immersion duration reduced the number of somatic embryos.
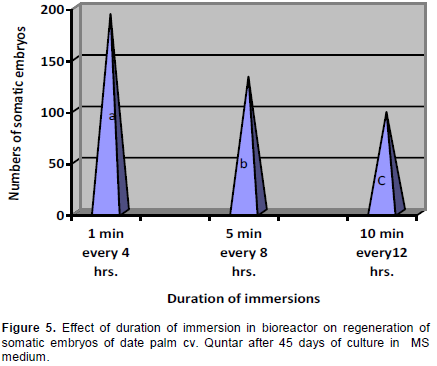
In culture systems with temporary tissue immersions, the immersion time was very important, since it determines nutrient and plant growth regulator uptake and can influence hyperhydricity (Etienne and Berthouly, 2002). Among the different immersion times used for formation of somatic embryos from suspension cultures, it was shown that the largest amounts of somatic embryos. The highest number of embryos were obtained using short immersions (Figure 5). Modifications of immersion frequencies and durations affect the efficiency of the somatic embryo regeneration (Albarran et al., 2005). Short immersions frequently repeated, 1 min immersions every 4 h, led to the largest quantities of torpedo-shaped embryos without hyperhydricity (Ducos et al., 2007). The adjustment of immersion time was critical with this type of bioreactor. According to Teisson and Alvard (1995) with 1 L temporary immersion bioreactors, 15 min’ immersion every 6 h led to successive development and germination of coffeeembryos, whereas for an identical culture medium, immersions of 1 min every 24 h halted embryo development and stimulated the production of adventive embryos. Besides, in an agitated liquid medium embryogenic callus disaggregated allowing the formation of suspension cultures. This confirms previous findings reported that immersion time, that is, duration or frequency, is the most decisive parameter for system efficiency, the temporary immersion system with other perennial crops which varied from 1 to 15 min immersion every 2 to 12 h (Etienne and Berthouly, 2002). Othmani et al. (2009) reported that the embryogenic callus of date palm cv. Deglet Bey turned brown and died using a RITA® bioreactor with immersion frequency of 5 min every 8 h. The growth is enhanced by forced aeration, since continuous provided sufficient oxygen supply to the tissue, which ultimately led to their faster growth. A similar result was obtained by Albarrán et al. (2005) who showed that short immersion periods (1 min every 12 h) strongly promoted the embryo production in coffee compared to long immersion duration (5 and 10 min every 12 hrs.). Also, this result was in accordance with Gatica-Arias et al. (2008) who showed that the highest somatic embryo of coffee development and fresh weight were obtained with 1 min of immersion every 8 h as compared of immersion with 5 and 10 min every 8 h.
Indirect shoot organogenesis from leaf derived callus
Effect of different treatments of plant growth regulators on a response of embryogenic
callus for shoots regeneration
Table 3 showed that from embryogenic callus (100 mg weight) derived from leaf base segments (white) gave the best significant response for shoot regeneration and number of shoot/100 mg weight when was cultured in MS medium supplemented with 3.0 mgl-1 2iP+1.0 mgl-1 NAA in comparison with other treatments, reached 58.34% and 8.28 shoots/100 mg weight, after 12 weeks from culture (Figure 6A, B). But, the embryogenic callus cultured in MS medium without hormone (control treatment) did not give any response for shoot regeneration.
The development of adventitious shoots from the undifferentiated callus masses
The process usually occurred after an intervening period of callus growth. Callus is differentiated and unorganized mass of parenchyma cells formed by the proliferation of parent tissue. Callus tissue is a good source of adventitious shoot regeneration )Dodds and Roberts, 1982). The presence of cytokinin along with auxin is necessary for shoot regeneration from callus derived from the leaf explants, a combination of auxin (1.0 mgl-1 NAA) and higher cytokinin (3.0 mgl-1 2iP) was the most effective (Table 3). The growth regulators added to medium play an important role in the growth and differentiation of explants (Tisserat, 1984). They might have more metabolically active cells with hormonal and nutritional conditions that are responsible for increased organogenesis (Famiani et al., 1994). The cytokinin-auxin combination has been widely used for organogenic differentiation in various protocols developed for date palm (AL-Mayahi, 2012).
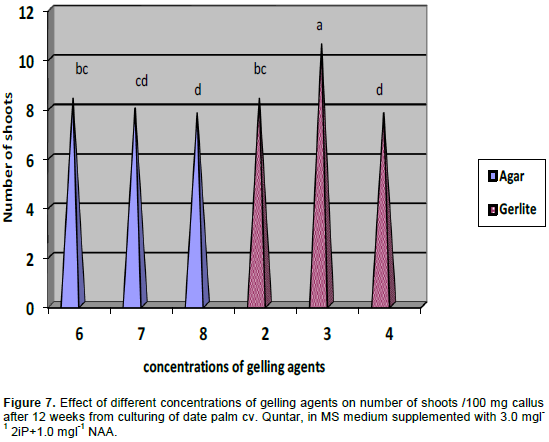
The emergence of the new bud from the primitive meristemic tissue is caused by the superiority of the cytokenines' balance over the auxines', inside the explant's cells, due to the successive cultivation in solution rich with the cytokenines. The importance of the cytokenines in releasing the process of meristems and, consequently, formation, is well-known (Auge, 1984), as well as the importance of the auxin and the cytokenines in enhancing the growth of the date palm buds (Amin, 2001). The formation of the organs in the moncotyledonae is, generally, enhanced by a big and sudden increase in the concentration of the cytokenines (Duhoux, 1988). Organogenesis can be induced by transferring callus or explants to a suitable medium or sequence of media that promote proliferation of shoot, root or both. For investigating the effects of different concentration of gelling agents "agar and Gelrite" on the shoot regeneration of date palm cv. Quntar, in MS medium supplemented with 3.0 mgl-1 2iP+1.0 mgl-1 NAA (Figure 7). The best result of shoot proliferation was resulted with 3 gml-1 Gelrite. Shoot organogenesis was found to be more efficient when Gelrite was used as the gelling agent as compared with agar. The superiority of Gelrite over agar for the purposes of shoot regeneration has also been reported for apple (Saito and Suzuki, 1999).
Histological investigations revealed the division and growth of callus cells continued for some time resulting in the enlargement of primary callus. Where active cell division occurred, certain cells of the meristems undergo divisions in such a way that, one product of a division becomes a new body cell, called derivative and the other remains in the meristem, called initials (Figure 8-1). Meristemoids formation could be detected on the top of the meristematic area on some peripheral callus sectors. They were constituted of densely cytoplasmic small cells with prominent, enlarged nuclei. Those meristemoids elongated and acquired a well- defined shoot primordia shape (Figure 8-2). The indirect formation of shoot buds from meristematic areas on callus surface. The vascular bundles were found initiated in the form of procambium, the cells of which assume a rather narrow elongated form because of the predominance of longitudinal division. The procambium then developed into distinct vascular elements, (Figure 8-3). The adventitious shoot with leaf primordial (L) and shoot meristems (MR) formed (Figure 8-4).
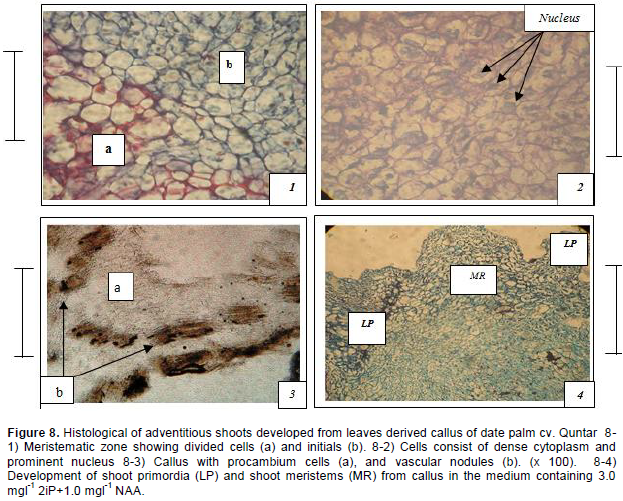
The emergence of the new bud from the primitive meristemic tissue and its transference into bud-generating-tissue, is caused by the superiority of the cytokenines' balance over the auxins. The hormonal balance of the solution made, apparently, the pro meristemic tissue continue their active division which led to an important increase in the mass of this pro meristemic tissue. Consequently, this tissue developed into primitive meristemic tissue, which is characterized by its rapid division and increase in size. The divisions led to the formation of meristematic areas as meristemoids. These consisted of small cells with a dense cytoplasm and prominent nucleus. These structures were more developed. Thus, the adventitious shoot buds were developed from meristemoid areas, as the result of the mitotic activity of cells. Meristemoids differentiated in the callus and are later transformed into cyclic nodules from which shoots or roots develop (Al-Khalifah and Shanavaskhan, 2012).
Elongation and rooting of regenerated shoots
The regenerated shoots, were separated and transferred to the elongation and rooting medium MS + 0.5 mgl-1 GA3 + 1.0 mgl-1 NAA (Figure 6C, D, E). The shoots rooted successfully (80%) with rapid elongation, after 6 weeks of culture (Figure 6D, E).
Acclimatization
The plantlets (produced from both two pathways), were acclimatized successfully in a mixture of peat moss and perlite (2:1), reached 80% after 10 weeks of transferred to plastic pots (Figure 6F). All the micropropagated plants were free from external defects.