ABSTRACT
White thread blight disease (WTBD) is currently emerging as an important foliar disease on cocoa in Ghana. The disease has been known in the country for many years. Yet, the incidence and severity levels on cocoa in the growing regions are not known. Surveys and sampling were conducted between 2011 and 2013 to estimate incidence and severity of WTBD in the six cocoa growing regions (Ashanti, Brong-Ahafo, Central, Eastern, Western and Volta) of Ghana. Diseased samples were assayed for the infecting fungus and its identification. Chi square tests were used to find relationships between age, sanitation practice and the disease severity. Effectiveness of chemical and cultural control methods against the disease were tested. The disease was found in all the cocoa growing regions of Ghana and out of 24,000 trees inspected, 1,281 (5.3%) were infected. The majority of infected trees (74.2%) were moderately affected but 3.2% of the trees were very severely affected and almost dead. A positive correlation (r = 0.889) was found between WTBD incidence and the severity. The most severely affected regions were Ashanti (13.8%), Brong-Ahafo (10.2%) and Western (7.6%) regions. Poor maintenance significantly (p=0.0001) increased the levels of disease occurrence and severity. Older cocoa trees also appeared more susceptible than younger ones. Pruning of affected branches controlled the disease better than fungicides spray. However, Nordox (75% copper (I) oxide) at 5 g/l and Metalm (12% metalaxyl and (60% copper (I) oxide) at 3.3 g/l fungicides were effective in reducing mycelial growth of the Marasmiellus fungus. Therefore, fungicide should be used in situations of severe infection to supplement pruning.
Key words: Thread blight, cocoa, Marasmiellus, disease severity.
Theobroma cacao L. (cocoa) is the source of chocolate, one of the world’s most popular foods. Ghana produces nearly 20% of the world total cocoa output contributing 8.2% to gross domestic product (GDP) and 30% of the total export earnings (Asante-Poku and Angelucci, 2013). Production in the country has recently seen appreciable increases from 450 000 tons in the year 2000 to 900,000 tons in 2010 and in excess of 1,000,000 tons in 2011 (FAO), 2012). However, Ghana’s average annual cocoa yield of 440 kg/ha as against potential yield of over 2.5 tonnes per hectare, is among the lowest in the world and compares unfavorably to important producers such as Cote d’Ivoire (580 kg/ha) and Indonesia (770 kg/ha). This is attributed largely to fungal diseases among several other important factors (Bailey et al., 2016; Wood and Lass, 1992). Cocoa tree is under constant threat from fungal pathogens where ever it is cultivated. The most important fungal disease of cocoa in Ghana is Phytophthora pod rot popularly known as “black pod disease” which can cause 100% yield loss (Dakwa, 1987). There are several other fungal diseases but most of them are considered less important because they apparently cause minor losses or are limited in distribution. One such disease which is increasingly emerging as a serious foliar disease of cocoa in the country is white thread blight disease (WTBD) caused by Marasmiellus scandens (Massee) Dennis & Reid [syn: M. scandens (Massee) (Opoku et al., 2007). The disease is worldwide in distribution and in the Amazon where cocoa tree originated, it has been reported on several important plant species including 18 native fruit trees (Gasparotto and Silva, 1999). The disease has wide host range and is found on economically important trees crops some of which are also found in cocoa farms as fruit crop or shade trees (Benchimol, et al., 2001).
The white thread blight disease derived its name from mycelial strands (threads) of the fungal pathogen that grow underneath cocoa branches, petioles and leaves causing leaf blight. Blighted leaves show distinctive brown to dark-brown decay followed by defoliation. Typically, defoliated leaves cling to each other and the tree from the mycelial strands of the fungus that can be seen on twigs and petioles (Opoku et al., 2007). Dead leaves with mycelia are major source of inoculum and are spread by wind, rain, insects, nesting birds and human activities (Kusunoki et al., 1997). The disease may be devastating when poor agricultural practices and favourable weather conditions prevail over time. In spite of reports of WTBD on cocoa in Ghana over the years, little advances have been made on the disease. Leston, (1970) reported white thread blight incidence of about 6-48% from four outbreak cocoa growing communities. Asare-Nyako (1987) studied the infection processes of the thread blight fungus on cocoa in the field, gauze house and laboratory. Scientific documentation regarding the disease incidence and severity levels on cocoa farms in Ghana is still lacking. This study was, therefore, carried out to document the occurrence and distribution of white thread blight disease on cocoa in all the growing regions of Ghana. It also assessed the relationships between the farm age, the farm sanitation level and the disease severity.
Disease assessment
Surveys were conducted between 2011 and 2013 cocoa seasons to assess incidence and severity of WTBD in the six cocoa growing regions (Ashanti, Brong-Ahafo, Central, Eastern, Western and Volta) of Ghana (Figure 1). Twenty farms were selected from each region and in each farm, 200 trees were visually inspected along a diagonal transect. The numbers of cocoa trees with WTBD symptoms were counted and the Disease Incidence (DI) estimated using the equation:
Disease Severity (DS) was estimated using a rating key similar to Akrofi et al. (2014) where;
None (0): No TBD symptom on tree canopy (values in parentheses are rating score).
Moderate (1): more than 25% but less than 50% of canopy infected.
Severe (3): more than 50% but less than 75% of canopy infected.
Very severe (5): more than 75% of canopy infected.
The DS was then measured based on a modified equation from that proposed by Kranz (1988);
Where,
Σ (a×b) = Sum of symptomatic trees and their corresponding score rating.
N = Total number of trees sampled
Sanitation levels in the farms were assessed based on visual observations and information from the farmers on their maintenance practices of weeding frequency, shade management, removal of mistletoe/epiphytes, infected fruits and over hanging branches. The farmers’ practices were scored on a 1-5 scale (1=none, 2=, incomplete, 3= infrequent, 4= complete, 5 =frequent) and the farms classified as either low in sanitation when there is no or incomplete and infrequent maintenance practice. High sanitation is when there is complete and frequent maintenance. The ages of the farms were recorded through interviewing the farmers.
Fungi isolation, identification and pathogenicity testing
Samples of cocoa leaves, twigs or branches showing signs and symptoms of white thread blight infection were collected from the surveyed farms for fungi isolation. Single strand isolations were made from the mycelial strands onto water agar plates and subsequently onto Potato Dextrose Agar (PDA) media. Pure cultures of the isolates were observed under light microscope (Leica, USA) and identification based on morphological characteristics using standard references (Singer, 1986; Humber, 2005; Kirk et al., 2008; Desjardin et al., 1993). Pathogenicity test was conducted on healthy cocoa leaves (3-months old) from mixed cocoa hybrids. The leaves were surface sterilised with 70% alcohol (Sigma-Aldrich, USA) and rinsed twice in sterile distilled water (SDW). Three, 10-mm mycelia discs of the test isolates were placed on abaxial and similarly on adaxial surface of leaves in completely randomised design with 5 replications. The leaves were incubated in aluminum tray (72 x 62 x 10 cm) lined with moist plastic foam (Latex foam, Ghana Ltd) on laboratory bench and examined after 7 days for infection and re-isolation of test isolates. The entire experiment was repeated thrice.
Evaluation of management practices
Partial systemic and contact fungicides which proved promising in a laboratory assay viz. Nordox (75% copper (I) oxide a.i., Nordox Industries, Oslo, Norway), Metalm (12% metalaxyl and 60% copper (I) oxide a.i., ALM International, South Africa) and Ridomil Gold (6% mefenoxam + 60% copper (I) oxide a.i., Ceiba Geigy Ltd., Basle, Switzerland) were used. The fungicides were also tested in combination with pruning of white thread blight fungus on naturally infected cocoa trees on farmer’s farm at Osino (6°20′84.4′′N, 0°29′64′′W) in the Eastern Region. The farm was selected because of the proximity to Cocoa Research Institute of Ghana to facilitate continuous monitoring and also to demonstrate to farmers in the locality, treatment options available to them. The treatments applied were:
1) Spraying infected branches at 4 weekly intervals.
2) Pruning and spraying of pruned surfaces at 4-weekly intervals
3) Pruning infected branches
4) Untreated (control) trees
Forty replicate trees were selected for each treatment during the peak disease period of August - November, 2014. Test fungicides were applied in 15 L volumes using pneumatic knapsack sprayer (MATABI®, Goizper S. Coop, Spain). Long handled pruning knife were used to prune affected branches. Treatment trees were assessed weekly and the mycelia growth measured with tape measure. Data obtained was expressed as growth rate per week and percent mycelial growth inhibition calculated for each fungicide.
Statistical analysis
Descriptive and inferential statistics were employed to analyse survey data using the Statistical Package for Social Sciences (SPSS) Version 10. For descriptive statistical techniques, frequency distribution and mean were computed to study the data. Chi-square test was used to infer relationships between the farm age, its sanitation and the disease severity. The farms were grouped into old and young for analysis. The productive age of the surveyed farms was taken as 40 years with 1-20 years as the most economic phase (that is, younger farm) and 21-40 as old farm. One way ANOVA with Duncan’s Multiple Range Test (SASS Program, version 22) was performed to compare the distribution of Disease Incidence (DI) and Disease Severity (DS) amongst surveyed regions. Test of normality was employed to determine whether DI and DS data should be transformed or not prior to analysis.
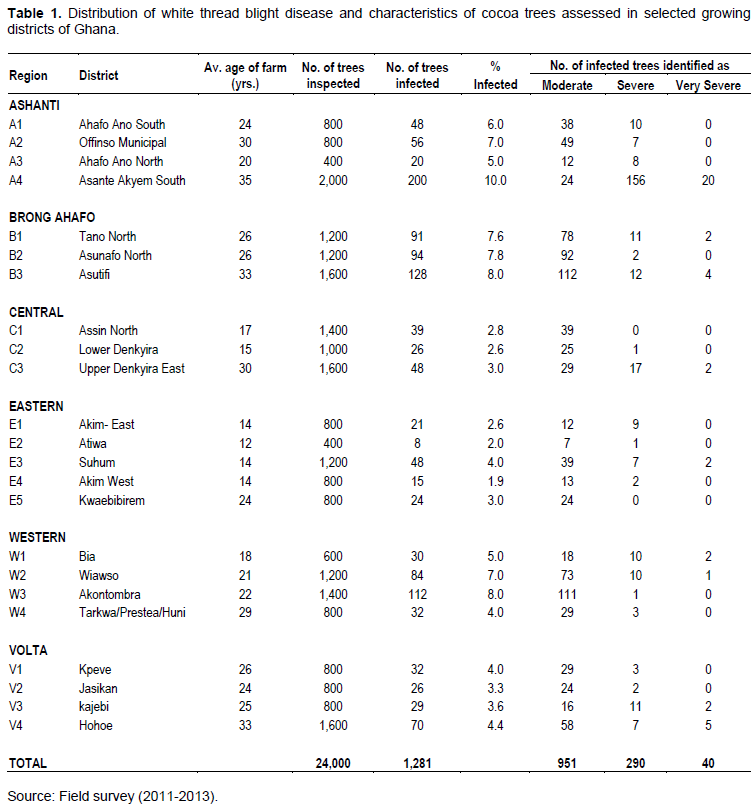
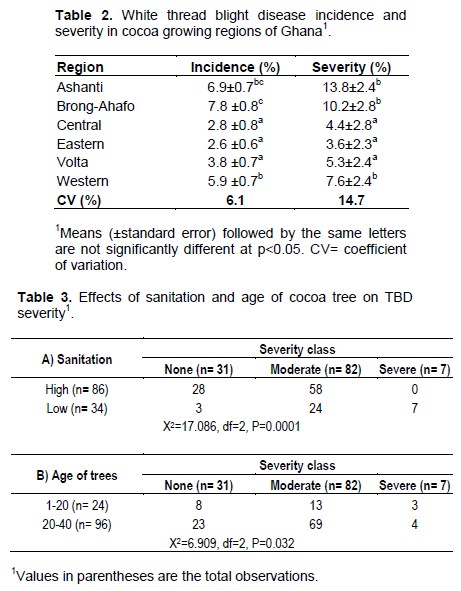
White thread blight disease was found in all the cocoa growing regions of Ghana (Figure 1). From the 24,000 trees aged between 12-35 years which were inspected nationwide, 1,281 of them representing 5.3% were infected (Table 1). Majority of the infected trees (951), were moderately affected and these constituted 74.2% infection. However, 40 (3.1%) trees were very severely affected with some of them completely killed (Table 1). Among the surveyed districts, the disease incidence (DI) ranged from 1.9% in Akim West of Eastern region to 10% in Asante Akyem south in the Ashanti region. The most severe forms of the disease were encountered in the Asante Akyem south district (Table 1). In the regions, disease incidence of 7.8% occurred in the Brong-Ahafo and it was significantly (p<0.05) higher than the other regions except Ashanti. In the Western region however, incidence levels were significantly (p<0.05) higher than Central, Eastern and Volta regions (Table 2). The most severely affected regions were Ashanti, Brong-Ahafo and Western. White thread blight disease incidence was found to correlate well (r = 0.889, y=1.517x) with the disease severity. From a total of 120 120 farms (20 farms from each of the 6 regions) surveyed, sanitation levels in 86 (71.6%) of them were high while 34 (28.4%) appeared low (Table 3a). There was highly significant (p=0.0001) relationship between the levels of sanitation and TBD occurrence and severity. Farms with high sanitation levels were either free of the disease or had low incidence and severity. The disease did not occur in 31 farms and 28 (90.3%) of them had high sanitation levels (Table 3a). Meanwhile, the sanitisation levels in all the farms which were severely affected were low. It was again observed that older cocoa trees (20+ years) were more susceptible (p=0.023) than younger ones (Table 3b). In the study, 24 young farms (1-20 years) and 96 older cocoa farms (20-40 years) were assessed for WTBD. Infection was recorded in 73 (76%) of the old farms and 16 (66.6%) of the young farms.
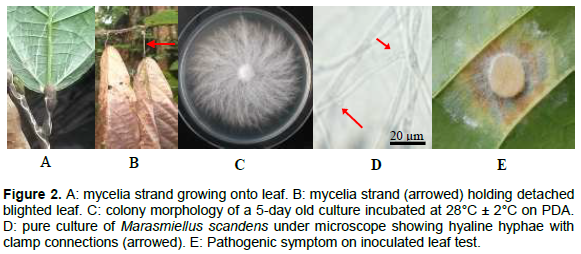
Pathogen isolation and identification
In the field, M. scandens on cocoa was seen as a network of web-like dried strands, predominantly, on the lower surfaces of leaves and undersides of branches. The strands always branch off from the petioles to leaves and then spread out into numerous fine ones (Figure 2A). The fine strands initiated dark-brown necrosis and as the whole leaf became involved, the leaf separated at the petiole but usually remained hanging from mycelial strand that grew over the petiole from the branch (Figure 2B). On agar plates, the fungus produced characteristic thread-like mycelia with more or less feathery margins (Figure 2C). The colonies were cottony white and produced stroma in abundance when kept under light (either continuous or alternate light/dark) but no fruiting bodies were observed. Hyphae were hyaline and contained numerous clamp connections that were identical to each other (Figure 2D). Pure cultures of the fungus infected cocoa leaves from the lower surface initiating necrosis similar to those observed in the field (Figure 2E). Pathogenicity was confirmed when the fungus was re-isolated from leaves with characteristic symptoms similar to those from the field.
Disease management
All the fungicides performed similarly against the WTBD disease (Table 4). Mycelia growth reduction achieved with pruning was 94% and was higher (p = 0.001) than the fungicides spray (22-30%). Combining pruning with fungicides application resulted in 92-100% growth reduction. Pruning combined well with fungicides, Nordox and Metalm, to completely suppress mycelia growth of the white thread blight fungi (Table 2). Metalm a partially systemic fungicide was effective at slightly lower dosage (3.3 g/L) when compared to the contact fungicide, Nordox (5.0 g/L).
White thread blight disease has been known on cocoa in Ghana for many years but few studies have been conducted on it since Leston (1970) drew attention to the potential threat of the disease to production. This study represents the first scientific information on regional distribution of abundance and severity of the disease on cocoa in Ghana. Previous studies noted M (Marasmius) scandens as the causal fungus of WTBD on cocoa in Ghana (Opoku et al., 2007; Asare-Nyako, 1987). This was confirmed in the current study where Marasmiellus Murill fungi isolates were associated consistently with the WTBD. They produced culture characteristics on PDA similar to those previously found on cocoa in Ghana. Necrotic symptoms development on healthy leaves after inoculation indicated that the isolates were pathogenic and their identities were confirmed as M. scandens (Massee) Dennis & Reid according to the original taxonomic work of Dennis and Reid (1957), then Singer (1986) and Desjardin et al. (1993). The disease is widespread on cocoa in Ghana with high incidence rates in the growing districts. Most farmers have limited knowledge about the disease and were therefore indifferent towards its control resulting in incidence levels which increasingly affected the disease severity. The correlation coefficient of relationship between the incidence and severity was close to 1 (r = 0.889). Therefore, it was not surprising that the survey data described the highest disease incidence and the most severe forms of the disease as both occurring in Asante Akyem South district of Ashanti region. Thus, supporting the assumption that disease incidence always create epidemiologically significant concept with its severity (Seem, 1984). The study also found a significant (p= 0.032) relationship between the disease severity and age of cocoa. Prevalence of the disease on older trees (20+ yrs) and its slow development on young ones suggests Marasmiellus fungi as weak pathogen which attacks maturing tissue. Susceptibility of plant tissue to diseases with age has been reported (Reuveni et al., 1986; Rodrigues, 1994). Hence, improved host plant nutrition which delays tissue maturation is important to boost cocoa trees resistance to WTBD. In the long term, replacement of old tree stock is recommended especially when cocoa yield decreases at an increasing rate over time despite the improved host nutrition.
The high WTBD incidence and severity in most cocoa growing regions is attributed largely to low adoption of farm sanitation practices. This is because the farmers hardly remove excess shade trees or prune overhanging and interlocking branches of the cocoa trees which create humid conditions that support fungi infection (David, 2005). The farmers also tend to disregard debris of blighted leaves on their trees which are the main source of inoculum that can initiate epidemics once favorable conditions for dispersal and infection prevail. The recommended cultural practice for efficient management of cocoa in Ghana requires frequent weeding (4 times/year where cocoa canopy is not closed), removal of mistletoes/epiphytes, excess shade or branches, basal chupons, infected and mummified pods (Akrofi et al., 2003). This package of farm sanitisation is an essential part of integrated black pod disease management and has been found effective against white thread blight infection. The disease occurrence was very low in farms maintaining high sanitation standards and the most severe forms were virtually absent from such farms. Pruning of white thread blight affected branches was the most effective way of managing the disease. However, re-growth of fungal strands on pruned branches was observed on some treated trees. This probably occurred when parts of the fungus mycelia remained on the trees. It will therefore require additional experiments to determine the most effective point below which infected branches should be pruned to make the practice efficient. Meanwhile, re-growth of the fungus was prevented when pruning was supplemented with copper-based fungicides spray (Nordox 75 WG and Metalm 72 WP). Therefore, fungicides should necessarily be included in the treatment of severely affected farms for effective control of the disease.
White thread blight disease has been known on cocoa in Ghana but essentially this is a first report of spread of the disease in all the cocoa growing regions in the country. Age of cocoa farm and the cultural maintenance practiced highly influenced the disease distribution and abundance. Integrated method of sanitation and chemical control of the disease has been demonstrated assuring farmers of effective management of M. scandens, causal fungus of white thread blight disease on cocoa in Ghana.
The authors have not declared any conflict of interests.
The technical support from staff of the Mycology Laboratory is duly acknowledged. This paper (CRIG/06/2015/041/002) is published with kind courtesy of the Executive Director of CRIG, Akim Tafo.
REFERENCES
|
Akrofi AY, Amoako-Attah I, Assuah MK, Kumi-Asare E (2014). Pink Disease Caused by Erythricium salmonicolor (Berk. & Broome) Burdsall: An Epidemiological Assessment of its Potential Effect on Cocoa Production in Ghana. J. Plant Pathol. Microb. 5:215.
Crossref
|
|
|
|
Akrofi AY, Appiah AA, Opoku IY (2003). Management of Phytophthora pod rot disease on cocoa farms in Ghana. Crop protection 22:469-477.
Crossref
|
|
|
|
|
Asante-Poku A, Angelucci F (2013). Analysis of incentives and disincentives for cocoa in Ghana. Technical Notes Series, MAFAP, FAO, Rome 44 p.
|
|
|
|
|
Asare-Nyako A (1987). White thread blight of cocoa. Proceedings of 1st Intl. Cocoa Pests and Disease Seminar, Accra – Ghana. pp. 132-138.
|
|
|
|
|
Benchimol RL, Poltronieri LS, Trindade DR, Albuquerque FC (2001). White-thread blight: Five new hosts in the state of Pará, Brazil. Fitopatol. Bras. 26:778-778.
Crossref
|
|
|
|
|
Bailey BA, Ali S, Akrofi AY, Meinhardt LW (2016). Phytophthora megakarya, a causal agent of black pod rot in Africa. In: Cacao Diseases: a history of old enemies and new encounters (Eds. Bryan Bailey and Lyndel Meinhardt). Springer International Publishing Switzerland pp. 267-303.
Crossref
|
|
|
|
|
Dakwa JT (1987). A serious outbreak of the black pod disease in marginal areas of Ghana. In: Proceedings of the 10th International Cocoa Research Conference, Santo Domingo, Dominican Republic pp. 447-451.
|
|
|
|
|
David S (2005). Learning about sustainable cocoa production: A guide for participatory farmer training. Integrated Crop and Pest Management. Sustainable Tree Crop program, IITA, Yaoundé, Cameroon.
|
|
|
|
|
Dennis RWG, Reid DA (1957). Some marasmioid fungi allegedly parasitic on leaves and twigs in the tropics. Kew Bulletin. 12(2):287-292.
Crossref
|
|
|
|
|
Desjardin DE, Gordon SA, Petersen RH (1993). Observations on two rhizomorph-forming species of Marasmiellus. Mycol. Res. 97:111–122.
Crossref
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAO) (2012). Cocoa beans production. Accessed April 2012, available at http://faostat.fao.org/
|
|
|
|
|
Gasparotto L, Silva SEL (1999). New hosts of Pellicularia koleroga in the State of Amazonas, Brazil. Fitopatol. Bras. 24:469.
|
|
|
|
|
Humber RA (2005). Fungal identification USDA-ARS Plant Protection Research 103 Unit US Plant, Soil & Nutrition Laboratory Tower Road Ithaca, New York.
|
|
|
|
|
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Dictionary of the Fungi (10th ed.). Wallingford, UK: CAB International P 402.
|
|
|
|
|
Kranz J (1988). Measuring Plant Disease. In: Experimental Techniques in Plant Disease Epidemiology, Kranz J. and J. Rotem (Eds.). Springer, Berlin, ISBN: 978-0-387-18128-8 pp.35-50.
Crossref
|
|
|
|
|
Kusunoki M, Kawabe Y, Ikeda T, Aosh K. (1997). Role of birds in dissemination of the thread blight disease caused by Cylindrobasidium argenteum. Mycoscience 38:1-5.
Crossref
|
|
|
|
|
Leston D (1970). Incidence of thread blight on cocoa in Ghana. PANS, 16(3): 516- 517.
Crossref
|
|
|
|
|
Opoku IY, Assuah MK, Domfeh O (2007). Manual for the identification and control of diseases of cocoa. CRIG Technical bulletin No.16, Akim-Tafo, Ghana.
|
|
|
|
|
Reuveni M, Tuzun S, Cole JS, Siegel MR, Kuc J (1986). The effects of plant age and leaf position on the susceptibility of tobacco to blue mold caused by peronospora tobacina Phytopathology 76:455-458.
Crossref
|
|
|
|
|
Rodrigues KF (1994). The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia 86:376-385.
Crossref
|
|
|
|
|
Seem RC (1984). Disease incidence and severity relationships. Ann. Rev. Phytopathol. 22:133-150.
Crossref
|
|
|
|
|
Singer R (1986). The Agaricales in modern taxonomy. 4th ed. Koeltz Scientific Books, Koenighstein Germany 981 p.
|
|
|
|
|
Wood GAR, Lass RA (1992). Cocoa. Tropical Agriculture Series, 4th Edition Longman Press, London pp. 338-340.
|
|