ABSTRACT
The objective of this research was to know the performance of the seeds from different species of the Leucaena genus (Leucaena leucocephala, Leucaena lanceolata, Leucaena diversifolia, Leucaena macrophylla and Leucaena esculenta), stored under ambient conditions and subject to an evaluation of their physical, physiological and sanitary quality. The material came from the harvests of the collection planted at the Pastures and Forages Research Station Indio Hatuey (Matanzas province, Cuba). Samples were taken from two seed lots, with 6 and 18 months of storage, in which the moisture content, germination and viability were determined. In addition, the hard, fresh and dead seeds were counted. A completely randomized design was used, and the data were processed through a variance analysis (ANOVA). The results indicated the variation, in terms of quality, of the stored seeds at 6 months with regards to those that had been stored for 18 months; however, both lots are considered adequate. The rotting increased (75%) with the months of storage; while the viability decreased as the storage time and physiological age increased (95 vs. 65,7% at 6 and 18 months, respectively). Fungi were the main microorganisms that caused seed rot, with higher effect on the seeds that had been stored for 18 months.
Key words: Physical and physiological quality, Leucaena spp., seeds, storage
The need to produce food for a constantly growing population causes large areas of forests and rainforests to be degraded, with which the biodiversity of these ecosystems is lost. In such areas there are tree and shrub species with potential to contribute to the livelihood” instead of economy of farmers. In this sense, Ramos-Quirarte et al. (2009) stated that trees and shrubs, due to the many products and benefits they provide, represent an important patrimony for the dwellers. Tree and shrub species are valuable resources for animal feeding and the wild fauna. Their use represents an option in livestock production systems, to avoid dependence on concentrate feeds (Suárez et al., 2011). In the tropic and subtropical regions the Leucaena leucocephala species is one of the most used in such systems, as well as in cut-and-carry systems for small farmers (Ruiz and Febles, 2006). However, its initial growth is slow and has some disadvantages regarding its establishment, due to the dormancy of seeds, which is caused by the presence of a cuticle impermeable to water and oxygen (Sánchez and Ramírez-Villalobos, 2006; González et al., 2009). The initial propagation of the species of leucaena is by sexual seed (Hughes, 1998), which is a practical and economic process, highly used by farmers. The physiological quality of the seeds of this species is determined by the germination percentage, moisture content and vigor, if it is considered that they are very important factors at plantation level (Román-Miranda et al., 2013).
In this sense, seed conservation (storage) constitutes the basis of breeding works and allows the exchange of germplasm and the preservation of genetic viability, mainly. However, despite this, there are few studies on all species of the genus. The main reason for seed storage according to reports by Besnier Romero (1989), is their distribution in time and space, which from the point of view of their utilization, for which they are reproduced, should allow their longevity; that is, the preservation of their adequate viability and vigor in the most appropriate places for their germination and establishment during a reasonable time.
In this sense, according to Alves et al. (2002), seed storage acquires high importance in the production process because there is generally a time interval between the seed harvest and the later seeding, which can last a few days or be extended for several months, according to the species and crop, production site, prevailing environmental conditions and production technology. The fundamental reason for storage is related to the preservation of the physiological and sanitary quality of the seeds, due to the reduction of contamination by potential insect pests, the incidence of microorganisms and the minimization of the deterioration rate.
During storage, seed deterioration cannot be prevented, although the rate of the process can be minimized through adequate procedures of production, harvest, drying, processing, transportation and through biochemical and physiological disturbances started right after the physiological maturation, which causes vigor reduction, ending in the loss of germination capacity. Among the factors that affect the maintenance of seed quality along a certain period, the humidity degree, condition of the storage environment (mainly air temperature and relative humidity) as well as the type of packing used stand out (Gálvez, 2012).
Nevertheless, in spite of the studies conducted, specifically with the species L. leucocephala, as well as of the importance of seed quality for farmers, there are limit studies about the performance of stored seeds; therefore, the objective of this research was to determine the performance in different species of the Leucaena spp. genus, stored at ambient temperature.
Provenance of the evaluated plant material
The seeds of the evaluated species of the Leucaena spp. Genus (L. leucocephala, Leucaena lanceolata, Leucaena diversifolia, Leucaena macrophylla and Leucaena esculenta), stored under ambient conditions with time difference of 6 and 18 months, came from the harvests of the collection planted at the Pastures and Forages Research Station Indio Hatuey –Matanzas province, Cuba.
Edaphoclimatic conditions
The mother plants from which the seeds were collected are planted on a humic nodular ferruginous hydrated lixiviated Ferralitic Red soil, of fast dessication, clayey and deep on limestone (Hernández et al., 2015). According to the above-mentioned author’s reports, this soil is slightly acid. The results of the chemical composition of the soil are shown in Table 1.
Climate characteristics
The climate of the site is classified as of tropical savanna, characteristic of Cuba (Academia de Ciencias de Cuba, 1989), in which the tropical marine conditions prevail with marked seasonality of the rainfall, where the influence of the arctic and continental polar air masses is felt in the winter dry season which is extended from November to April. In the last 15 years before the research, the annual average temperature of the zone was 24.3°C, July was the warmest month, with 28.6°C, and January the coldest one, with 20.6°C. During the study the maximum temperatures reached 33.4°C in August and the minimum ones went down to 14.2°C in January. The average sum of the annual rainfall was 1 331.18 mm, with the highest value in June (235,8 mm) and the lowest one in February (only 27.4 mm). The rainfall during the rainy season (May-October) represented, as average, 79.8 % of the total annual volume. The evaporation in the zone increased since January, with maximum values in April (220 mm). The annual average relative humidity was 82.6%, with the highest value in July (89.0%) and the lowest one in April (75.5%).
Post-harvest seed management
The pods were manually harvested. Afterwards, the ginning and natural drying (72 h under sunlight) of the seeds was carried out, in order to reduce their moisture content between 10 and 13%, with which, according to Gálvez (2012), the growth of fungi and, thus, the destruction of embryos, is avoided. Then, they were processed and stored (6 and 18 months) under ambient conditions, during the two years of research, in a rectangular shed of 52 m2 of basis and 3 m of height, with continuous adequate ventilation, according to the regulations for this type of facility (Harrigton, 1972; Gálvez, 2012). The seeds were selected according to ISTA rules (2005), and the physical and physiological quality of the seeds with 6 and 18 months of storage, through three of the recommended essays; in addition, the sanitary quality was evaluated. The seeds were not treated.
Determination of the physical and physiological quality of the seeds
To determine the humidity content (%), the gravimetric method in hot stove (130 ± 30°C) was used, according to the recommendation made by ISTA (2005); the calculation was made based on the fresh weight. Four replications of 10 g of seeds from each lot were used, and the formula proposed by ISTA (2005) was applied. The germination test (%) was performed according to ISTA (1999) standard for tropical trees using four replicates of 100 seeds for each species evaluated. The emission of the radicle from the seed coats was taken as germination criterion (ISTA, 2005). The seedlings were evaluated after planting and were classified from the criterion of the seedling evaluation handbook, according to ISTA rules (2005). The hard, fresh and dead seeds were also counted. The result was expressed in percentage of normal seedlings. To corroborate the accuracy of the results, the tolerance table 14.3 of chapter 14 of the Handbook of Seed Technology for Genebanks was used (Ellis et al., 1985). In the case of dead seeds, the ones damaged by fungi or other microorganisms and the rotten ones without visible signs or symptoms of affectation were included. The viability test was estimated through the biological method recommended by ISTA (1999), thus determining the viability percentage, which was expressed in viable and non-viable seeds.
Statistical analysis
The data expressed in percentages were transformed using the mathematical function sen-1√% (Steel and Torrie, 1992). They were processed by a one-way variance analysis (ANOVA), through the InfoStat program. The means were compared by Duncan’s test (1955), at 5% level of significance, after verifying that they fulfilled the fit of normal distribution and variance homogeneity.a
Regarding the moisture content, there were no significant differences between both lots (Table 2). All the seeds did not respond equally to storage, independently from the fact that there were no significant differences among the species: the seeds of L. esculenta showed lower moisture content, followed by those of L. lanceolata, with regards to the ones of L. leucocephala, at 6 as well as at 18 months.

The storage of leucaena seeds in the tropic is carried out under environmental conditions of high relative humidity (85% of relative humidity) and temperature (> 26°C), which are not controlled; these associated variables significantly influence quality. Bustamante (2010) reports that humidity contents have direct influence on seed longevity, because they stimulate the metabolic activity of the embryo. This circumstance is relevant because the physiological quality of the seed is negatively affected, while what is required is high physiological potential represented in seed vigor. The concepts of ‘vigor’, attribute belonging to the seeds capable of germinating, and ‘deterioration’, are physiologically linked and are reciprocal aspects which have incidence on seed quality. Deterioration has a negative connotation, while vigor has an extremely positive meaning: vigor decreases as deterioration increases due to the rise of temperature and relative humidity (RH) in the storage time. Deterioration refers to the ageing process and death of the seeds, and thus, vigor is the main component of quality that is affected by the deterioration process (Teofilo et al., 2004).
Seed longevity is given by a balance between intrinsic and extrinsic factors that mainly affect the deleterious mechanisms of metabolism and the repair processes. Likewise, the period in which the seeds remain viable is very variable and is genetically determined, although the storage conditions and environmental factors have a determinant effect on the duration of a seed’s life (Bustamante, 2010). Leucaena seeds are small; for such reason they can register differences in the absorption of water from the prevailing RH in the storage medium, which can originate variations in the moisture contents of the samples. The hygroscopic nature of the seeds and the environmental conditions under which they are kept influence the process of water uptake or loss; this causes damage which reduces the seed physiological quality. That is why, as the hydration and water loss cycles increase, germination decreases and the effects are more critical with the hydration periods (Aramendiz-Tatis et al., 2007). The increase of RH and temperature, associated with the storage time of the seed, leads to a progressive decrease of seed vigor due to the deterioration caused by the loss of membrane integrity (Delouche et al., 1973). The RH exerts influence on the moisture content of the seed and its effect on their longevity is direct. In this regard, Powell and Matthews (1981) express that seed ageing occurs much faster when they show high moisture content and are stored at high temperature, because the biochemical processes are affected (Amaral and Lemos, 2009).
While most of the cultivated and wild plants maintain their viability better when they are preserved with low moisture contents and at low temperatures, others, especially those from tropical regions, do not survive when that content is lower than a certain value (Gálvez (2012). For such reason, he distinguishes two seed categories, according to their response during storage: orthodox and recalcitrant. This aspect was also approached by Schmidt (2000), who places the species of the Leucaena genus in the first category. Its seeds can be satisfactorily kept exsitu, during long periods, under adequate conditions; they can be dried to low moisture levels, without suffering damage; and their longevity is increased with the decrease of humidity and temperature. According to Harrington (1972), in seeds stored under ambient conditions, the moisture content is considered optimum when it reaches between 10 and 12%; which coincides in a certain way with the results of this study. Likewise, Benkonva and Zakóva (2009) state that such values are appropriate for short- and medium-term conservation. On the other hand, Delouche et al. (1973) reports that with these values the physiological deterioration will depend directly on other processes inherent to the seeds (for example, the biochemical ones such as reactivation and synthesis of enzymes, respiration, absorption of O2, consumption of carbohydrates, among others), and not on the moisture content itself. Similarly, Gálvez (2012) states that there should be a balance between the relative moisture of the seeds and that of the environment; however, it is valid to point out that the seeds with hard coats, such as those of Leucaena, are an exception.
The physiological quality of a seed lot implies that they fulfill the indispensable condition of viability; but, in addition to being alive, they must germinate and produce a seedling with its essential structures correctly developed. To start the germination process, they must be physiologically mature and, thus, the maturity status is considered an important characteristic of their quality. For such reason, the immature or not completely mature seeds, generally, show lower physiological quality with regards to the seeds that reached maturity (Del Valle, 2008). In this sense, significant differences (p ≤ 0.05) were found in germination (Table 3); there was a higher percentage in the seeds with lower storage time (6 months). A marked difference was also observed in the performance of L. esculenta (65 %) with regards to L. leucocephala, (between 80 and 85 %) which is perhaps conditioned by the more frequent use of the latter in breeding programs, or because it is more domesticated than the former. On the other hand, the 18-month seeds showed high percentages of hard, fresh and dead seeds (Figure 1); unlike the ones with lower physiological age (15 and 7 %, respectively). The germination test provides sufficient information on the performance of a seed lot. Although there are many factors that can affect germination and, thus, seedling emergence, temperature plays an important role (Nacimento, 2005; Aramendiz-Tatis et al., 2007). However, authors such as Lopes and Pereira (2005) agree that there is not a general optimum temperature, because each species has a particular optimum temperature range to germinate, and within that range marked differences can appear among cultivars and, seemingly, among species.


Physiological age is another intrinsic factor which can affect good germination; hence the 6-month seeds had a better performance in this indicator than the 18-month ones. According to reports by Courtis (2013), as they advance in age, seeds tend to absorb water faster. This phenomenon is considered associated to the loss of integrity of cell membranes. Another factor that could have influenced the decrease of germination is the maturity status of the seeds, which can be visually inferred through the observation of the absence or presence of chlorophyll pigments, which act as receptors of the light energy in the photosynthesis process. Nevertheless, in different situations they can be transformed into a negative factor, by reducing the germination capacity and the vigor of seeds (Scheeren and Tolentino, 2005). During the period of seed formation, and even after the plants reach the status of physiological maturity and of commercial maturity, the tissues of the pods and the coats of the seeds are contracted and elongated as a result of the humidity and temperature fluctuations that occur throughout the day (Arango et al., 2006). This hydration and dehydration process which occurs in the seed structures, during seed development, originates damages of different magnitude and affects, to a higher or lesser extent, the physiological quality. In this test the superiority of the L. leucocephala seeds with regards to those of L. esculenta was also observed, which could be conditioned by the higher use of the first species compared with the others; as well as by the presence of hard seed coats (Gálvez, 2012), which can cause some species to be harder than others; although, in general, the values were low. The L. esculenta seeds were the most affected ones in the higher storage time.
In this sense, another factor that perhaps influenced the decrease of germination (Figure 2) is seed rot, which reached the highest values in the seeds with more time of storage and physiological age (75% of affectation). Román-Miranda et al. (2013) obtained similar results in seeds of L. lanceolata. In tropical countries, where temperature and RH always reach high and continuous values, the presence of potentially pest insects and microorganisms is favored. Thus, according to studies conducted by Cerovich and Miranda (2004) for good storage under ambient conditions, it is essential to maintain the moisture content of grains and seeds low. According to reports by the mentioned researchers, this high affectation may depend more on the relative humidity and temperature of the environment, than on the moisture content of the seeds. In addition, the moisture content the seeds of other species of the genus should have in order to be stored is not accurately known. The highest affectations occurred in the L. esculenta seeds. This superiority could have been influenced by the storage period, which was evidently higher at 18 months, and also by the environmental conditions; and to a lesser extent, the affectations provoked by other causes appeared; in them it was observed that although there were no significant differences, there was an increase in the seeds with higher physiological age.

Along with the above-mentioned factors, others should also be considered that somehow have incidence on seed storage, such as: genetic characteristics of the species to be stored, pre-harvest history of the crop, structure and chemical composition of the seed, degree of maturity, presence of dormancy, vigor and mechanical damage (Cerovich and Miranda, 2004). Similarly, the performance of the hard, fresh and dead seeds may not be due only to the depletion of nutritional reserves –which they preserve mostly, even after the loss of their germination capacity–; but also to the damage suffered by the cell membranes as well as the membranes of intracellular organelles (mitochondria, plastids, among others), which is known as natural aging during storage under ambient conditions, among other causes (Jaramillo et al., 2012). In this sense, Bustamante (2010) states that seed deterioration is a complex mechanism of which all the physiological and biochemical processes that participate in it are not accurately known; while the compensation of the damage undergone in storage occurs during imbibition, which causes the delay of germination and vigor loss or, even, if the damage is very important, the seeds are incapable of germinating or the seedling dies after germination. The viability percentage (Table 4) decreased as the storage time increased (95 vs. 65,7 %, for the seeds with 6 and 18 months, respectively), which coincides with the report by Benkonva and Zakóva (2009) about the trend of this quality indicator during the storage. In this case, the seeds of L. esculenta were also the most affected.
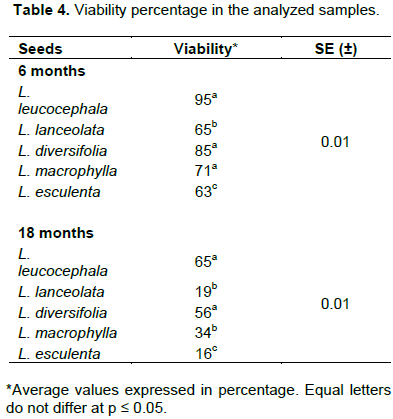
The viability loss that occurred in the 18-month seeds could have been due to the action of fungi or other microorganisms. According to Gálvez (2012), when this occurs other processes are affected, such as: enzymatic activity; synthesis or metabolism of proteins, carbohydrates or lipids; cell respiration; and increase of the volatile components which are toxic for the seeds, the chromosomal material and the DNA synthesis. This deterioration does not occur in a uniform way, but, in general, starts in meristematic areas, especially in the root meristem. Likewise, it depends on each species and the group to which it belongs (recalcitrant or orthodox seed). Equally, although it was not considered in the research, the inadequate collection processing and transportation (to the storehouse, until the moment of seeding) could have influenced the decrease or loss of viability. In general, the seeds showed an acceptable performance during the study, if it is considered that the increase of relative humidity and temperature, associated to the storage time, lead to a progressive decrease of vigor, due to the deterioration caused by the loss of membrane integrity (Delouche, 2002). It is important to mention that in the consulted literature there are very few studies about the seeds of species of the Leucaena genus: only some accessions of L. leucocephala are mentioned, while regarding the others their use as parents in breeding programs is reported (L. diversifolia and L. macrophylla).a
(1) There was variation in terms of the physical, physiological and sanitary quality in both lots; although it was more marked in the one with 18 months of storage. The most affected were the seeds of the species L. esculenta.
(2) There was an increase in seed rot as the storage period under ambient conditions increased; the most affected were the ones of the species L. esculenta.
The authors have not declared any conflict of interests.
REFERENCES
|
Academia de Ciencias de Cuba (1989). Nuevo Atlas Nacional de Cuba. Instituto Cubano de Geodesia y Cartografía. La Habana, Cuba. p. 41.
|
|
|
|
Alves E, Paula R, Oliveira A, Bruno R, Diniz A (2002). Germinação de sementes de Mimosa caesalpiniaefolia Benth. em diferentes substratos e temperaturas. Rev. Bras. Semen. 24(1):169-178.
|
|
|
|
Amaral F, Lemos N (2009). Condiciones ambientales de almacenamiento para conservación de semillas de especies ortodoxas. SEEDnews P. 4.
|
|
|
|
Aramendiz-Tatis H, Cardona C, Jarma A, Robles J, Montalván R (2007). Efectos del almacenamiento en la calidad fisiológica de la semilla de berenjena (Solanum melongena L.). Agronomía Colombiana 25(1):104-112.
|
|
|
|
Arango MR, Salinas AR, Craviotto RM, Ferrari SA, Bisaro V, Montero MS (2006). Description of the environmental damage on soybean seeds (Glycine max (L) Merr). Seed Sci. Technol. 34:133-141.
Crossref
|
|
|
|
Benkonva M, Zakóva M (2009). Seed germinability of selected species after five and ten years storage at different temperatures. Agriculture (Pol´nohospodárstvo) 55(2):119-124.
|
|
|
|
Besnier Romero F (1989). Semillas. Biología y tecnología. Mundi_prensa. Madrid.
|
|
|
|
Bustamante J (2010). Calidad física y fisiológica en semillas de híbridos de maíz de los Valles Altos Centrales de México y su relación con el establecimiento en campo. Tesis presentada como requisito parcial para obtener el Título Académico de Maestra en Ciencias. Colegio de Postgraduados. Montecillo, Texcoco 108 p.
|
|
|
|
Cerovich M, Miranda F (2004). Almacenamiento de semillas: Estrategia básica para la seguridad alimentaria. Revista Digital del Centro Nacional de Investigaciones Agropecuarias de Venezuela. CENIAP HOY. N° 4 enero-abril 2004. 8p.
|
|
|
|
Courtis AC (2013). Guía de estudio de Fisiología vegetal. Germinación de semillas. Cátedra de Fisiología Vegetal. UNNE. 22 p.
|
|
|
|
Del Valle C (2008). Calidad fisiológica y efecto de la presencia de semillas verdes de soja (Glycine max (L.) Merr) en lotes destinados a simiente Tesis para optar al Grado Académico de Máster en Ciencias Agropecuarias. Mención: Tecnol. Sem. 134 p.
|
|
|
|
Delouche JC (2002). Germinación, deterioro y vigor de semillas. SEEDnews 6(6). En:
|
|
|
|
Delouche JC, Matthes RK, Dougherty GM, Boyd AH (1973). Storage of seed in subtropical and tropical region. Seed Sci. Technol. 1(2):671-700.
|
|
|
|
Duncan DB (1955). Multiple range and multiple F test. Biometrics 11:1.
Crossref
|
|
|
|
Ellis RH, Hong TD, Roberts EH (1985). Handbook of Seed Technology for Genebanks. International Borrad for Plant Genetic Resources, Roma, Italia 210 p.
|
|
|
|
Gálvez C (2012). Almacenamiento y conservación de semillas. Material vegetal de Reproducción, Manejo, Conservación y Tratamiento. 18 p.
|
|
|
|
González Y, Reino J, Machado R (2009). Dormancia y tratamientos pregerminativos en las semillas de Leucaena spp. cosechadas en suelo ácido. Pastos Forrajes 32(4):1-1.
|
|
|
|
Harrington JF (1972). Seed storage and longevity. In: Seed biology. (T. T. Kozlowski, Ed.). Academic Press. New York and London 3:145.
Crossref
|
|
|
|
Hernández A, Pérez J, Bosch D, Castro N (2015). Clasificación de los suelos de Cuba. Instituto Nacional de Ciencias Agrícolas. Instituto de suelos. Ministerio de la Agricultura. AGRINFOR. La Habana, Cuba 93 p.
|
|
|
|
Hughes CE (1998). Leucaena. Manual de Recursos Genéticos. No. 37. Oxford Forestry Institute. Department of Plant Sciences. University of Oxford P 91.
|
|
|
|
ISTA (2005). International Seed Testing Association. International rules for seed testing association. Bassersdorf, Suiza. 500 p.
|
|
|
|
ISTA (1999). International rules for seed testing. Seed Sci. Technol. 27:302.
|
|
|
|
Jaramillo A, Martínez M, Cardozo C, Burgos J (2012). Determinación de condiciones controladas de almacenamiento seguro para semillas de portainjertos de lima ácida 'Tahití' Rev. Corpoica Cienc. Tecnol. Agropecu. 13(2):151-158.
Crossref
|
|
|
|
Lopes JC, Pereira MD (2005). Germination of cubiu seeds under diferent substrates and temperatures. Rev. Bras. Semen. 27(2):146-150.
Crossref
|
|
|
|
Nacimento WM (2005). Vegetable seed priming to improve germination at low temperature. Hortic. Bras. 23(2):211-214.
|
|
|
|
Powell AA, Matthews S (1981). Evaluation of controlled deterioration, a new vigour test for small seeds vegetables. Seed Sci. Technol. 9(3):633-640.
|
|
|
|
Ramos-Quirarte A, Aguirre A, Medina RF, López LF, Camarillo UFJ (2009). Evaluación de plantas arbóreas asociadas con pastos para sistemas silvopastoriles en la región central de Nayarit. Rev. Comput. Prod. Porcina 16(1):59-63.
|
|
|
|
Román-Miranda ML, Martínez-Rosas LA, Mora-Santacruz A, Torres-Morán P, Gallegos-Rodríguez A, Avenda-o-López A (2013). Leucaena lanceolata S. Watson ssp. lanceolata, especie forestal con potencial para ser introducida en sistemas silvopastoriles. Rev. Chapingo Serie Cienc. For. Ambient. pp. 103-114.
|
|
|
|
Ruiz TE, Febles G (2006). Agrotecnia para el fomento de sistemas con leguminosas. Parte 2. En: Recursos Forrajeros Herbáceos y Arbóreos. (Ed. Milagros Milera). EEPF "Indio Hatuey" Matanzas, Cuba-Universidad de San Carlos de Guatemala, Guatemala p. 103.
|
|
|
|
Sánchez PY, Ramírez-Villalobos P (2006). Tratamientos pregerminativos en semillas de Leucaena leucocephala (Lam.) de Wit. y Prosopis juliflora (Sw.) DC. Rev. Fac. Agron. (LUZ) 23(3):257–272. Obtenido de.
View
|
|
|
|
Scheeren BR, Tolentino CF (2005). La baja calidad de semillas verdosas de soja. Seednews. Edición noviembre-diciembre 2005 pp. 22-23.
|
|
|
|
Schmidt L (2000). Guide to handling of tropical and subtropical forest seed. (Ed. K. Olensen). Danida Forest Seed Centre, Denmark P 511.
|
|
|
|
Steel RGD, Torrie JH (1992). Bioestadística: principios y procedimientos. Segunda edición. Mc Graw – Hill/Interamericana de México, S. A. 622 p.
|
|
|
|
Suárez A, Williams LG, Trejo C, Valdez HJI, Cetina AVM, Vibrans H (2011). Local knowledge helps select species for forest restoration in a tropical dry forest of central Veracruz, México. Agrofor. Syst. doi: 10.1007/ s10457-011-9437-9.
|
|
|
|
Teofilo EM, Oliveira S, Esmeraldo AM, Madeiros S, Barbosa FD (2004). Qualidade fisiológica de sementes de aroreira (Myracrodruon urundeuva Allemao) em função do tipo de embalagem, ambiente e tipo de armazenamento. Rev. Cienc. Agron. 35(2):371-376.
|