ABSTRACT
The soil-surface dwelling invertebrate assemblage of four sites (habitat patches) in Luchaba Nature Reserve was assessed using pitfall traps. A total of 335 specimens in three phyla (Arthropoda, Annelida and Mollusca) were sampled. Of the nine arthropod orders recorded, four were identified to seven families and ten species while five orders and two phyla (Annelida and Mollusca) were separated into 15 morphospecies. The eucalypt site supported fewer taxa compared to indigenous acacia and grassland patches while the mixed alien patch attracted the highest numbers of invertebrate families, species and individuals. Although species composition across sites was not significantly different (P>0.05), specimen counts showed significant differences (P<0.05). The implications of these preliminary results suggest that habitat-patch level management for conserving action in the short term should consider eradicating the species-poor eucalypt stands from the reserve while replacing all alien plants in the reserve area with native flora in the medium to long term. Furthermore, widespread/abundant species that occurred in all four sites e.g. Crematogaster sp, Pardosa crassipalpis and Pheidole sp. and habitat-restricted taxa can be used as potential bio-indicators for assessing the conservation value of habitat patches in Luchaba Nature Reserve and other protected areas of the King Sabata Dalindyebo Municipality.
Key words: Soil-surface dwelling invertebrates, nature reserve, indigenous plants, alien invasive plants.
Anthropogenic activities tend to accelerate the problem of alien invasions, which in turn affect agriculture, forestry and human health, resulting in biotic homogenization worldwide (Richardson and van Wilgen, 2004; Pimentel et al., 2005; Usio et al., 2009). Apart from the impact on human communities, invasive alien plants are responsible for the local extinction of many indigenous species in South Africa (Samways et al., 1996; Magoba and Samways, 2008), and regarded as the second major threat (after habitat destruction) to the biodiversity of any particular area (Richardson and van Wilgen, 2004; Macdonald et al., 2003; Olckers and Hulley, 1991).
Theoretically, it is widely expected that invasive plants, simply by occupying a large amount of space, impose a significant impact on the native vegetation and their associated food webs (Gerber et al., 2008). Several studies suggest that invasive plant species generally harbour smaller herbivore assemblages than native plant species (Gerber et al., 2008; Mgobozi et al., 2008). Three theories expounded by Tallamy (2004) explain this scenario. The first one predicts that specialists herbivores should be unable to grow and reproduce on plants with which they share no evolutionary history, the second one predicts that the energy stored by alien plants is not available to indigenous specialist and thus unavailable to higher trophic levels that include the insects in their diets, and the third predicts that these plants may not be palatable to most native insects. Given that there is strong association between most arthropods and native vegetation or the microhabitat it creates (Mgobozi et al., 2008; Olckers and Hulley, 1991), any decrease, extinction or alteration of the physical characteristics of some native plant species or habitats after alien plant colonization may negatively impact on species-specific herbivores (Palmer et al., 2004; Pauchard and Alback, 2004).
Protected areas (nature reserves) in South Africa remain critically important refugia that provide high quality habitat patches for invertebrate biodiversity conservation even though challenges resulting from their size and number do arise. Moreover, most of the country’s rich biodiversity lies outside of the approximately 6% of land area under protected area systems (Turpie, 2004; Blanchard and Holmes, 2008), with its native and semi-natural ecosystems also under increasing threats from alien plant invasions (Nel et al., 2004; Gorgens and van Wilgen, 2004; Olckers and Hulley, 1991). Healthy biological communities depend principally on interactions among small organisms (mostly invertebrates and microbes) (Hartley and Rogers, 2010). Over 4.7% of formally protected land in the Eastern Cape Province of South Africa is now biologically invaded, and this proportion is increasing (Masubelele et al., 2009; Foxcroft et al., 2011).
The topography of the Eastern Cape Province ranges in elevation from 0 to 1500 m a.s.l along a 200 km E-W transectin one latitude. The ecology of this area is influenced by montane climate and moist savannah vegetation type at higher elevations, and Afromontane forest with sub-tropical climate along the eastern and southern coasts of the province.
Reserves and non-formally protected areas/landscapes of the King Sabata Dalindyebo Municipality (KSDM) of the Eastern Cape fall within the Albany Centre of Endemism. Although these landscapes are increasingly under threat from local endemic plant extinctions resulting mainly from overgrazing, agriculture and alien plant invasions (Smith and Wilson, 2002), they are nevertheless growing in significance as elements of the matrix where indigenous biodiversity conservation action must be undertaken. However, little is known about the habitat-level impact of invasive alien and native plant cover on soil-surface dwelling invertebrates within protected areas of the municipality at a local spatial scale. The overall aim of this initial study therefore was to determine the response of invertebrates to various types of vegetation cover in Luchaba Nature Reserve(LNR) under the following specific objectives: i) identify the soil-surface-dwelling invertebrate assemblage of the reserve, ii) assess the ecological impact of invasive alien and indigenous plant cover on faunal species composition.
Study Site
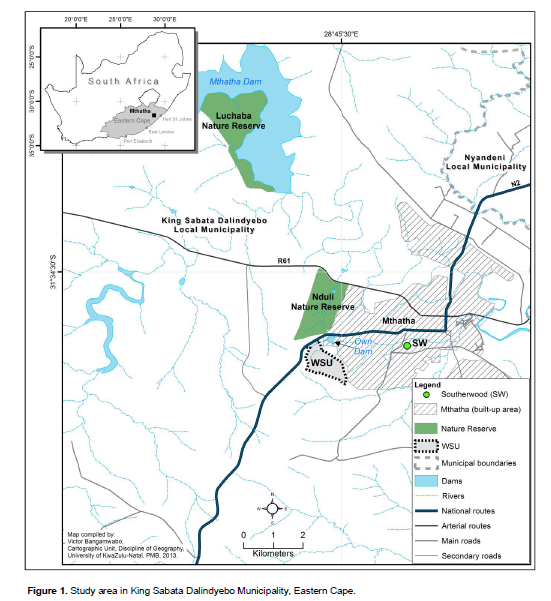
The study was carried out in Luchaba Nature Reserve (LNR) (460 ha) situated at 31°35’S, 28°45’E and 758 m a.s.l in the King Sabata Dalindyebo Municipality (KSDM) of the Eastern Cape Province of South Africa (Figure 1). This reserve is an un-proclaimed protected area on state land, managed as a nature reserve by the Operations Directorate of the Eastern Cape Parks Board (ECPB). Climate is characterised by mean winter and summer temperatures of 13 and 26°C respectively, with mean annual precipitation of 634 mm (DWAF, 2005). Natural forest in the reserve is made up of indigenous trees e.g. Acacia karroo (Hayne), Acacia sieberiana DC., Acacia xanthophloea B., Erythrina caffra Thunb. and Zanthoxylumcapense (Thunb.) Harv. (Moll, 1981; Palgraves, 2002). Common grass species are Eragrostis curvula (Schrad) Nees, E. Plana Nees, E. racemosa (Thumb.), Paspalum dilatatum Poir, Themeda triandra Forssk and Pennisetum Rich species while invasive alien plant species present in the reserve comprise of Eucalypt, black wattle, Lantana camara L., bugweed and inkberry. The geology of the reserve comprises predominatly of shales and sandstones of the Beaufort series of the Karoo system. These landforms are interlaced with dolerite dykes (Acocks, 1988).
Sampling site design and description
Four sites (habitat patches) that varied in structural and compositional vegetation cover were selected a-priori from a 1500 m2 land surface in the reserve. Each site measured 30 m2 and was stratified into four sampling units (Sus), each Su measuring 5 m2, separated from each other by about 10 m. Percentage estimates of total surface area of Su covered by dominant vegetation types were determined as follows.
(i) Eucalypt plantation (EU) patch with over 90% eucalypt tree cover
(ii) Mixed alien (MA) patch with about 60% cover of Lantana camara (L.), Black Wattle and Bug weed, and 30% cover of native herbaceous vegetation,
(iii) Indigenous acacia (IA)patch with about 90% indigenous acacia trees interspersed with indigenous herbaceous plants, sedges and grasses.
(iv) Indigenous grassland (IG) patch comprising of over 70% grasses, sedges and about 20% herbaceous vegetation cover.
Invertebrate sampling
Two pitfall traps were placed in each Su to capture invertebrate specimens. Traps consisted of 250 ml blue plastic cups with rim diameter of 75 mm and were sunk into the ground with rim openings maintained at the same level with the ground surface. The cups were three-quarter filled with a mixture of soapy detergent and water as a trapping medium, and were then left open in the ground for 24 h to capture soil-surface dwelling invertebrates. Specimens were collected from 32 traps during each of three sampling occasions making 96 records units in all. The sampling technique was according to Southwood and Henderson (2000), and designed to maintain sample independence as soil-surface dwelling invertebrate species are much less mobile or dispersive. Specimens were sorted from other flying arthropods in the traps, preserved in vials with 70% ethanol, and transported to the laboratory for preliminary identification. Specimens were identified using a Zeiss Stereo dissecting microscope Model STEMI DV4 and a field guide by Picker at al. (2004). Spider identities were confirmed by a taxon specialist at the Agricultural Research Council (ARC) Plant Protection Research Institute (PPRI) in Pretoria, and thereafter using reference works by Dippenaar-Schoeman and Jocque (1997). Ants were identified at the Biosystematics Division of the Agricultural Research Council (ARC), Pretoria while other insects were identified using Carruthers (2008) and Picker et al. (2004). Unidentified (morphospecies) were coded, preserved in 70% alcohol for future identification by taxon specialists. Invertebrate specimen data was collected during species-rich summer months of April (weeks two and three) and May (week 1) in 2011.
Statistical analysis
Data sets were collated for each sampling unit (Su) and arranged in data matrices as proposed by Ludwig and Reynolds (1988) and Clarke and Gorley (2006). The statistical software programs DIVERSE and CLUSTER in PRIMER V6 (Clarke and Warwick, 2001) were used to determine indices of diversity and classification of species data respectively. Species-by-sample unit data matrices were 4th root transformed to balance rarer and common species. The Bray-Curtis measure of similarity (Bray and Curtis, 1957) was then applied to the data to generate sampling unit similarity matrices that were fused successively using group average linking. Results describing patterns obtained using clusters were represented by a dendrogram. Species rank (k-dominance curves) for all four sites were calculated using the programme DOMPLOT (PRIMER V6). Curves extracted information on patterns of relative species abundances without reducing that information to a simple numeric diversity index (Lambshead et al., 1983; Clarke and Gorley, 2006). All invertebrate abundance data across sites was long-transformed to maintain normality and to satisfy the requirement for ANOVA.
Taxonomic profile of invertebrates sampled in Luchaba Nature Reserve
Even though flying arthropod species e.g. flies, wasps and butterflies were also collected in pitfall traps across habitat patches during the study period, these arthropod taxa were not included in the analysis as pitfall trapping was not the conventional method for sampling them. A total of 335 soil-surface dwelling invertebrate specimens in three phyla (Arthropoda, Annelida and Mollusca) were sampled. Of the nine arthropod orders sampled, four were identified to seven families and ten species while the remaining five orders and two phyla (Annelida and Mollusca) were separated into 15 morphospecies. Invertebrate species sampled across the four habitats showed varying degrees of richness and abundance patterns in response to habitat variables such as vegetation structure, composition and disturbance gradients associated with alien and indigenous cover. The highest number of invertebrate species and individuals were recorded at the mixed alien site while the Eucalypt site had the least number of specimen counts (Table 1, Figure 2). Species rank abundance (K-dominance) curves showed that the mixed alien site had a greater species evenness trend than the other three sites while the eucalypt patch had the highest species dominance with Pardosa crassipalpis and Pheidole spp having above 59% level of dominance (Figure 3a).
The dendrogram (Figure 3b) showed that the mixed alien and indigenous acacia sites had a high percentage similarity at above 80% in terms of species composition and distribution patterns. The orders with most abundant invertebrates individuals were the Araneae, and the Hymenoptera. There were no statistically significant differences (P>0.05) among habitat patches in terms of number of faunal species recorded. However, differences in total number of individuals (N) were significant (P<0.05). Also, there were statistically significant differences in abundance among sites for Araneae (P<0.01), suggesting that the population of this species-rich faunal group responded significantly to habitat patch heterogeneity. In addition to the Araneae, other non-insect arthropods sampled included morphospecies belonging to the orders Diplopoda, Opiliones, Isopoda and morphs of the phyla Annelida and Mollusca.
Epigaeic invertebrate distribution patterns habitat patches
Species sampled across the four habitat patches showed varying degrees and patterns of response to habitat patch characteristics e.g. structure, composition and disturbance gradients associated with invasive and indigenous plant cover. Some groups of invertebrates were probably not affected and/or responded slowly to the presence of invasive vegetation than other groups. The phylum Arthropoda was the most diverse, dominated by ants and beetles while non-insect arthropods were represented by spiders, isopods and centipedes. The phylum Mollusca was represented by Valloiniasp. belonging to the family Valonidae while only two morpho-species of the Annelida were sampled. Even though there was no significant difference in number of species sampled across the four habitat patches, the eucalypt patch was more uniform in vegetation structure and composition, attracting the least number and abundance of invertebrate species. Faunal abundance at this site was highly attributed to the fact that a few species e.g. Crematogaster sp and P. crassipalpis were recorded in high numbers. The other patches were more heterogeneous in vegetation structure and complexity. This scenario probably accounted for greater habitat quality associated with faunal richness and abundance especially at the mixed alien patch.
Sensitivity of invertebrate taxa at sites and conservation implications
Morphospecies of the orders Diplopoda, Opiliones, Isopoda, and the two phyla (Annelida and Mollusca) were sensitive to different habitat types, with the mixed alien and indigenous acacia habitats harbouring a majority of these taxa, and therefore capable of providing ideal and optimal habitat conditions for conserving them. The Coleoptera are known to utilize most trophic niches and comprise about 40% of all insect species (Stork, 1990; Desender et al., 1991; Koch et al., 2001). In this study, the order was represented by the Caminara sp. (Carabidae) and Aphodius sp. (Scarabaeidae), and were found to be site-specific, occurring in low populations only at the mixed alien and indigenous vegetation patch.
Spiders (Araneae) and ants (Formicidae) have also been used extensively for invertebrate biodiversity conservation assessments in South Africa due to their comparatively low dispersal abilities and therefore their great potential for use as indicators of habitat quality (Lovell et al., 2010; Muelelwa et al., 2010; Parr and Chown, 2001; Dippenaar-Schoeman and Craemer, 2000). Members of this group responded to habitat conditions at varying degrees across sites e.g. P. crassipalpis was relatively abundant, occurring across all sites as potential habitat indicator species worthy of conservation as a common/widespread species in the reserve area while Xysticus natalensis was habitat-specific, and restricted to the mixed alien patch.
Even though maximum invertebrate species richness is not always reached at undisturbed sites, each group displays a specific pattern (Palmer et al., 2004). The mixed alien site attracted the highest number of invertebrate taxa sampled during the study, supporting the finding by Harris et al. (2004) that the impact of invasive plants on native biodiversity is not always negative. Furthermore, biodiversity estimators indicate that undisturbed habitats can be less diverse than invaded habitats (Palmer et al., 2004). The study clearly showed that the indigenous acacia and indigenous grassland sites harboured comparable numbers of invertebrate taxa and specimen counts that were higher than that found in the eucalypt patch, but lower than counts made at the mixed alien patch. Widespread/abundant species that occurred throughout all four habitat patches e.g. Crematogaster sp, P. crassipalpis and Pheidole sp as well as habitat-restricted species e.g. X. natalensis (Table 1) can be used as potential indicators for assessing the conservation value of habitat patches in Luchaba Nature Reserve and other protected areas of the King Sabata Dalindyebo Municipality. However, more data on a broader spatial and temporal scale is needed to support species response patterns reported in this preliminary study, since responses of soil-surface dwelling invertebrate assemblages to invasive alien and native plant cover in the reserve may likely be species-specific.
Most invertebrate species were sampled in the mixed alien and indigenous acacia patches that showed a high level of ecological similarity (Figure 3b) suggesting that habitat patch level management for conservation action in the short term should consider eradicating the species-poor eucalypt stands from the reserve while replacing all alien plants in the reserve area with native flora in the medium to long term.
The authors have not declared any conflict of interest
Funding for this project was provided by the National Research Foundation (NRF) and the Directorate for Research Development at Walter Sisulu University. We thank the Eastern Cape Parks Board and the Luchaba Nature Reserve management for logistical support and granting us permission to undertake the study at the reserve.
REFERENCES
|
Acocks JPH (1988). Veld types of South Africa. Memoirs of the Botanical Survey of South Africa 57:1-146. |
|
|
Blanchard R, Holmes PM (2008). Riparian vegetation recovery after invasive alien tree clearance in the Fynbos Biome. South Afr. J. Bot. 74:421-431.
Crossref |
|
|
Bray JR, Curtis JT (1957). An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 27:325-349.
Crossref |
|
|
|
Carruthers V (2008). The wild life of Southern Africa. The larger illustrated guide to the animals and plants of the region. Struik Publishers, Cape Town. |
|
|
|
Clarke KR, Gorley RN (2006). PRIMER V6:User Manual/Tutorial. PRIMER-E Ltd, Plymouth, U.K. |
|
|
|
Clarke KR, Warwick RN (2001). Change in Marine Communities: An Approach to statistical Analysis and Interpretation, 2nd ed. Plymouth Marine Laboratory, UK: PRIMER-E LTD. |
|
|
|
Desender K, Maelfait JP, Baert L (1991). Carabid beetles as ecological indicators in dune management (Coleoptera: Carabidae). El. Supplement 5:239-47. |
|
|
|
Dippenaar-Schoeman AS, Craemer C (2000). The South African national Survey of Arachnida (SANSA). Plant Protect. News 56:11-12. |
|
|
|
Dippenaar-Schoeman AS, Jocque R (1997). African spiders: an identification manual. Plant Protection Research Institute. Agricultural Research Council (ARC). Pretoria. Handbook 9. |
|
|
|
DWAF (2005). A woodland strategy framework for the Department of Water Affairs and Forestry (DWAF), Pretoria. |
|
|
|
Foxcroft LC, Jarosik V, Pysek P, Richardson DM, Rouget M (2011) Protected area boundaries as filters of plant invasions. Conserv. Biol. 25:400-405. |
|
|
Gerber E, Krebs C, Murrell C, Moretti M, Rocklin R, Schaffner U (2008). Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and Invertebrate assemblages in European riparian habitats. Biol. Conserv. 141:646-654.
Crossref |
|
|
|
Gorgens AHM, Van Wilgen BW (2004). Invasive alien plants and water resources in South Africa: current understanding and predictive ability and research challenges. South Afr. J. Sci. 100:27-33. |
|
|
|
Harris RJ, Toft RJ, Dugdale JS, William PA, Rees JS (2004).Insect assemblage in a native (Kunzeaericoides) and an invasive (Ulexeuropaeus) shrub land.New Zealand J. Ecol. 28:35-47. |
|
|
Hartley MK, Rogers WE (2010). Comparison of arthropod assemblages on an invasive and native trees: abundance, diversity and damage. Arthropod-Plant Interact. 4:237-245.
Crossref |
|
|
Koch SO, Chown SL, Davis AIV, Endrody Y,van Jaarsveld AS (2001). Conservation strategies for poorly surveyed taxa: a dung beetle (Coleoptera, Scarabaeidae) case study from Southern Africa. J. Insect Conserv. 4:45-56.
Crossref |
|
|
Lambshead PJD, Platt HM, Shaw KM (1983). The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J. Nat. Hist. 17:859-874.
Crossref |
|
|
Lovell SJ, Hamer ML, Slotow RH, Herbert D (2010). Assessment of sampling approaches for multi-taxa invertebrate survey in a South African savanna-mosaicecosystem. Austral Ecol. 35:357-370.
Crossref |
|
|
|
Ludwig JA, Reynolds JF (1988). Statistical ecology: a primer on methods and computing. John Wiley & Sons, New York. |
|
|
|
Macdonald IAW, Reaser JK, Bright C, Neville LE, Howard GW, Murphy SJ, Preston G (2003). Invasive alien species in Southern Africa. Global Invasive Species Programme. National Reports and Directory of Resources, Cape Town. |
|
|
Magoba RN, Samways M (2008). Restoration Ecology: Restoration of Aquatic Macro invertebrate Assemblages through Large-scale Removal of Riparian Invasive Alien Trees. J. Insect Conserv. 14(6):627-636.
Crossref |
|
|
Masubelele ML, Foxcroft LC, Milton SJ (2009). Alien plant species list and distribution for Camedeboo National Park, Eastern Cape, South Africa. Koedoe 51:515-525.
Crossref |
|
|
|
Mgobozi MP, Somers, MJ, Dippenaar-Schoeman AS (2008). Spider responses to alien plant invasions: the effect of short- & long-term Chromolaenaodorata invasion and management. J. Appl. Ecol. 45:1189-1197. |
|
|
|
Moll EJ (1981). Palgraves trees of Southern Africa. Struik Publications. Cape Town. |
|
|
Muelelwa MI, Foord SH, Dippenaar-Schoeman AS, Stam EM (2010). Towards a standardized and optimized protocol for rapid assessments; spider species richness and assemblage composition in two savanna vegetation types. Afr. Zool. 45:273-290.
Crossref |
|
|
|
Nel JL, Richardson DM, Rouget M, Mgidi TN, Mdzeke N, Le Maitre DC, van Wilgen BW, Schonegevel L, Henderson L, Neser S (2004). A proposed classification of invasive alien plant species in South Africa: towards prioritizing species and areas for management action. S. Afr. J. Sci. 100:53-64. |
|
|
|
Olckers t, hulley pe (1991). Impoverished insect herbivore faunas on the exotic bugweed Solanum mauritanium Scop. Relative to indigenous solanum species in Natal/KwaZulu and the Transkei. J. Entomol. Soc. S. Afr. 54:39-50. |
|
|
|
Palgraves KC (2002). Trees of Southern Africa Random House, Struik Publications. |
|
|
Palmer M, Linde M, Pons GX (2004). Correlational patterns between invertebrates composition and the presence of an invasive plant. ActaOecologica 26:219-226.
Crossref |
|
|
Parr CL, Chown SL (2001). Inventory and bioindicator sampling: Testing pitfall and Winkler methods with ants in a South African savanna. J. Insect Conserv. 5:27-36.
Crossref |
|
|
Pauchard A, Alback PB (2004). Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of South-Central Chile. Conserv. Biol. 18:238-248.
Crossref |
|
|
|
Picker M, Griffiths C, Weaving A (2004). Field guide to insects of South Africa. Struik Publishers. |
|
|
Pimentel D, Zuniga R, Morrison D (2005). Update on the environmental economic costs associated with alien-invasive species. Ecol. Econ. 53:273-288.
Crossref |
|
|
|
Richardson DM, van Wilgen BW (2004). Invasive alien plants in South Africa: how well do we understand the ecological impacts? South Afr. J. Sci. 100:45-52. |
|
|
Samways MJ, Caldwell PM, Osborne R (1996). Ground-living invertebrates assemblages in native, planted and invasive vegetation in South Africa. Agric. Ecosyst. Environ. 59:19-32.
Crossref |
|
|
|
Smith N, Wilson SL (2002). Changing land use trends in the Thicket Biome: Pastoralism to game farming, terrestrial Ecosystems Research Unit (TERU). Nelson Mandela Metropolitan University (NMMU), South Africa. Report No. 38. |
|
|
|
Southwood tre, henderson pa (2000). Ecological methods. 3rd Edition Blackwell Science. |
|
|
|
Stork NE (1990). The role of ground beetles in ecological and environmental studies. Andover, UK: Intercept. |
|
|
Tallamy DW (2004). Do alien plants reduce insect biomass? Conserv. Biol. 18:1689-1692.
Crossref |
|
|
|
Turpie JK (2004). The role of resource economics in the control of invasive alien plants in South Africa. South Afr. J. Sci. 100:87-93. |
|
|
Usio N, Kamiyama R, Saji A, Takamura N (2009). Size-dependent impacts of invasive alien crayfish on a littoral marsh community. Biol. Conserv. 142:1480-1490.
Crossref |