ABSTRACT
Maize (Zea mays L.) possesses high productive potential, excellent chemical composition and nutritive value, is one of the most important cereals in the world. This work aimed to evaluate the biochemical changes in two cultivars of maize, BRS Planalto and BR5202 Pampa, exposed to different amounts of nitrate and glutamine. The seeds were sown in polyethylene pots acclimated in a greenhouse, where began treatments with nitrate and glutamine at different doses (0.00, 0.10, and 10.00 mM) in five plots a completely randomized statistical design with six replicates. At 30 days, a single collection of material was performed and evaluated: chlorophylls a, b and total, carotenoids, total soluble carbohydrates, starch, sugars reducers and non-reducers, soluble protein and activity of nitrate reductase in leaves and roots. The content of photosynthetic pigments showed differences only between the doses tested. In general, an increase in nitrate concentration and a decrease in starch content, total soluble sugars, reducers and non-reducers for both cultivars. Comparing the effects of different sources and levels of N, the cv. BRS Planalto presented the greatest levels of total soluble proteins in leaves compared to cv. BR5202 Pampa. There was a decline in the activity of RN in both genotypes in response to high doses of N. Overall, there was no significant difference between N sources used. Nitrate reductase activity is greater than the 0.1 mM of nitrogen source, and is greater in leaves in relation to the roots, and glutamine does not inhibit its activity.
Key words: Zea mays L., nitrate reductase, nitrogen fonts.
Maize (Zea mays L.) is a monocot of the Poaceae family (formerly Gramineae) and is part of a group of plants that perform C4 photosynthetic metabolism (Marenco and Lopes, 2009). Due to high productive potential, excellent chemical composition and nutritive value, maize is one of the most important cereals grown and consumed in the world, and in many countries of Africa, Latin America and Asia, maize the food base of the population (Castro et al., 2009).
The culture of corn requires large amounts of nitrogen and usually requires the use of nitrogen fertilizer (organic and/or chemical) in coverage to complement the amount provided by the soil. However, the efficiency in the use of available nitrogen in the fertilizer is generally low which seems to depend on, among other factors, the concentration of the mineral in the soil and losses by leaching process (Figueiredo et al., 2005).
Studies reveal different responses regarding the time of application and nitrogen sources (Lara Cabezas et al., 2005). However, there is a high demand for nitrogen in the early stages of development (Silva et al., 2005). The importance of nitrogen known for duties in the metabolism of plants, participating as a constituent of proteins, cytochromes and chlorophylls, among others, in addition to being considered one of the most relevant factors for the increase in production by influencing the rate of emergence and expansion of foliar area (Taiz and Zeiger, 2009).
According to Marenco and Lopes (2009), nitrogen is preferentially absorbed by the roots of plants in the form of nitrate and ammonia. Nitrate can originate from organic matter mineralization that contains amino acid nitrogen which undergoes several biochemical transformations, but can also be of fertilizers containing such salt. Once absorbed by the cell, nitrate is reduced to nitrite by nitrate reductase (NR) and then to ammonium chloride by nitrite reductase (NIR). The ammonium chloride is immediately assimilated through the joint action of the enzymes glutamine synthetase (GS) and glutamate synthetase (GOGAT). The process of reduction and assimilation of N can occur on leaves and/or roots, simultaneously or not, between these organs, according to the species (Pate, 1980) and with environmental conditions (Costa, 1986).
The first step of this process is the reduction of nitrate to nitrite in the cytosol by the enzyme nitrate reductase (Taiz and Zeiger, 2009). Thus, NR is of fundamental importance in providing nitrogen to the plant. This enzyme has several factors that regulate its activity. The presence of nitrate itself induces its activity in various tissues. Light stimulates the protein levels of NR (Li and Oaks, 1994), as well as the presence needed in large quantity of reducing agents.
Glutamine synthetase is another important enzyme in the process of incorporation of nitrogen because it catalyses the step key of inorganic nitrogen assimilation: incorporation of ammonium chloride to glutamate which results in glutamine. Thus, the synthesis of glutamine by GS is considered a key process for plants to express their maximum productive potential (Unno et al., 2006). Glucose and starch products of the Calvin cycle which comes from the photosynthetic activity also stimulate the accumulation of NR (Lillo, 2004). However, Touraine et al. (2001) found that amino acids repress nitrate absorption.
Based on this context, this work aimed to evaluate the biochemical changes in plants of two cultivars of maize (Z. mays L.) exposed to different amounts of nitrate and glutamine in order to increase the knowledge about the influence of these sources of nitrogen for vegetable growth and to test its viability for modern agriculture.
Study area
The experiment was performed in the greenhouse of the Department of Botany, the Institute of Biology - IB of Universidade Federal de Pelotas - UFPel, Rio Grande do Sul, Brasil located at 31°46'19"S, 52°20'34"W with a height of about 7 m above sea level. The weather according to Köppen classification is the CFA type, that is, moist temperate weather with a warm summer and average rainfall of 1200 mm per year. The average temperature in the warmest month is 23.3°C and in the coldest 12.2°C. The average relative humidity is 78%.
Material and preparation
For this experiment, seeds of corn (Z. mays L.) cv. BRS Planalto and cv. BR5202 Pampa were used, provided by Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA, Clima Temperado, Pelotas, Rio Grande do Sul). These varieties were selected for being precocious and both possess tolerance to cold weather in southern Brazil and have been developed for the practice of family agriculture which is quite common in this part of the country.
The seeds (3 seeds/pot) were sown in polyethylene pots with a capacity of 1 L containing washed sand as substrate. At 3 days after emergence (DAE), scraping was carried out so that only one plant remained per pot. Plants remained in the greenhouse with an average daily temperature of 30°C ± 2°C. The plants were irrigated with water whenever required until 10 DAE.
The experiment constituted six treatments, three being different concentrations of nitrate and three different concentrations of glutamine (0.00, 0.10 and 10.00 mM), divided into five instalments, applied with 20 mL of each solution at intervals of 3 days in each treatment. Potassium nitrate was used as a source of nitrate.
Among the applications of the solutions, nitrogenized was provided a nutritive solution without nitrogen at DAE, a single material collection was carried out for the evaluations by biochemical analyses.
Biochemical analysis
For the biochemical analyses, samples of ~100 mg of the tissues of roots and leaves were collected, weighed and conditioned in containers of suitable solutions according to the extractions and maintained at temperatures of -20°C subsequent for processing. Analyses were carried out of photosynthetic pigment content (chlorophylls a, b and total, and carotenoids), starch, total soluble sugars, reducing and non-reducing sugars, total protein and activity of NR.
Chlorophylls (Chla and Chlb, total) and carotenoids (Car)
The procedure began with the maceration of the samples in the dark with addition of 80% acetone until a uniform extract was obtained. The extract centrifuged at 3000 rpm for 5 min and then the supernatant was collected and its volume was made up to 25 mL with 80% acetone. Absorbance of the samples was read in a spectrophotometer at 470, 645 and 663 nm according to Arnon (1949). The contents of chlorophyll a, b and total, and carotenoids were calculated according Lichtenthäler (1987).
Starch
Starch content determinations were carried out with centrifuged residuals after extraction of soluble carbohydrates according to the method described by Mc Credy et al. (1950).
Total soluble carbohydrates, reducing sugars and non-reducing sugars
The levels of total soluble carbohydrates were determined by means of reactions with Antrona according to Clegg (1956) and reducing sugars by the method of Somogy-Nelson (Nelson, 1944; Somogy, 1952). The non-reducing sugars were estimated by the differences between the concentrations of total soluble and reducing sugars. All results are expressed in mg.g MF-1.
Total soluble proteins
The samples was macerated with NaOH to 0.1 M and then centrifuged at 3000 rpm for 5 min. The supernatant was collected and the final volume was measured. From these, we collected a sample of 100 µL and added 5 µL Coomassie Blue. This mixture was stirred in the vortex for 2 min. The absorbance was measured in the spectrophotometer at 595 nm and the content is expressed in mg.g MF-1.
Nitrate reductase activity - NR
To quantify the in vivo activity of nitrate reductase, tissue samples from leaves and roots were used with modifications according to Cataldo et al. (1975). Samples were weighed and incubated in 0.1 M buffer phosphate-K (pH 7.5), 0.02 M KNO3, 2% propanol and 5% Triton X100. Subsequently, the material was filtered in vacuum desiccator in the dark for 5 min followed by incubation in a water bath at 30°C for 30 min in the dark to avoid the formation of reduced ferredoxin and to measure the nitrite formed before its reduction to ammonium. 2.0 mL of samples, 0.3 mL of 1% sulfanilamide in 3 M HCl and 0.3 mL of 0.02% N-naphthalene diamine hydrochloride was added. The total volume was made up to 4 mL with deionized water. The solution remained at rest for 10 min and was read in a spectrophotometer at 540 nm (Kenis et al., 1992). The activity is expressed in µmol NO2- g-1 MF h-1.
Statistical analyses
The experimental design utilized was completely randomized with six replications. The experimental unit constituted of a pot containing a plant. The effects of nitrogen levels were analysed by the Tukey test at 5% of probability using the program ASSISTAT (Silva, 1996; Silva and Azevedo, 2002, 2006, 2009).
In this present study, no significant differences were found in the contents of pigments between cultivars BRS
Planalto and BR5202 Pampa. However, significant differences in the contents of a, b and total chlorophylls were obtained in treatments with GLN and nitrate at a concentration of 10 mM compared to the control and the lowest dose (0.1 mM) which did not differ among themselves (Table 1) in both cultivars.
The carotenoid content of cv. BRS Planalto was significant in relation to the other treatments only at a dose of 10 mM. On the other hand, the levels of carotenoids in cv. BR5202 Pampa were significantly higher than in treatments with 0.1 mM nitrate and 10 mM GLN. Corn plants subjected to higher doses have generally higher content of pigment in relation to the lower doses. Majerowicz et al. (2002) also observed an increase in the contents of pigments in seven varieties of corn in response to an increase in N rates (15). The two genotypes used showed a 100% increase in chlorophyll levels a and b when the total dose of GLN increased from 0.1 to 10 mM (100-fold).
The content of photosynthetic pigments was sufficient to indicate the difference of the nutritional status of the plants and the efficiency of N rates utilized but not to differentiate between the behaviour of each cultivar. In the same way, the contents of chlorophyll showed the rates effect of N without producing differences between the maize genotypes tested (Majerowicz et al., 2002).
The concentration of starch in cv. BRS Planalto was significantly higher than the control only at a dose of 0.1 mM nitrate (Table 2). In cv. BR5202 Pampa, the starch content did not differ between the doses of 0.1 mM nitrate and GLN but at 10 mM both of nitrate and GLN were lower than the control.
Possibly this fact occurred due to large amount of GLN acting as your enzyme inhibitor preventing its action. Oliveira et al. (2013) stated that the glutamine synthetase is important in the absorption of N, and Hirel et al. (2007) claimed that the presence of N is crucial to grain filling which results in higher levels of starch and sugar.
For total soluble sugars, there was a difference for 0.1 mM nitrates, 0.1 mM and 10 mM GLN relative to the control for BRS Planalto. However, BR5202 Pampa did not show the same differences in soluble sugar levels compared with the control. The availability of N has affect the levels of soluble sugars from the roots, what happens in function of N is required (Table 3), to produce enzymes responsible for the carbohydrate synthesis which may be affected by the availability of N (Alfoldi et al., 1992).
In the cv. BRS Planalto, the content of reducing sugars was significantly different at doses of 0.1 mM of nitrates and 10 mM of GLN as compared to control. In the cv. BR5202 Pampa, there was a decrease in the level of reducing sugars with an increase in the two sources utilized. In both cultivars, the non-reducing sugar content was significantly higher than the control only for the treatments with GLN (Table 2).
In general, we observed that with an increase in the nitrate concentration, there was a decrease in the starch content, total soluble sugars, reducing and non- reducing sugars for both cultivars (Table 2). Probably, the increase in nitrate concentration affected the production of these organic compounds in the development stage analysed. Touraine et al. (2001) stated that there should be availability of carbohydrates in roots so that activity in the transporters of nitrate can occur. In this way, it appears that 0.1 mM nitrates can be considered sufficient for the genotypes and stage analysed.
For both genotypes analysed, starch content decreased with the increased availability of N. This shows that the starch can be a source of carbonic skeletons for incorporation of the N, when available in larger concentrations. The synthesis of starch is altered by N availability in plants (Medici, 2003). Carbon which is required during nitrate assimilation in many cases is obtained by slowing the rate of accumulation of starch or by means of starch remobilization (Scheible et al., 1997).
The ratio between carbohydrates and proteins in the leaves of cv. BRS Planalto showed the highest values when GLN was used as a source of N. There were differences between the sources of N and between GLN and control, when the ratio increased (Table 3). This may be due to the decrease in protein (Figure 1) or an increase in the level of total soluble carbohydrates (Table 2).
This demonstrates that the plant manages to make available the N absorbed as GLN and can incorporate it into protein molecules. The source of N available for the production of biomolecules is important for the growth of plants and can be distinguished by patterns in the metabolism of nitrogen and carbon (Britto and Kronzucker, 2002).
The cv. BR5202 Pampa had significant differences in the relationship between carbohydrates and proteins of leaves at 0.1 mM nitrate and GLN relative to the control and 10 mM. Meanwhile, in the roots of cv. BR5202 Pampa, there was no difference between the control and 0.1 mM nitrate. While the ratio of the total was similar, between the control and 0.1 mM of nitrate and GLN, there was a decrease in the concentration of leaves and roots.
The relationship between the shoot dry matter aerial part (DMA) and the root dry matter (DMR) was similar for both cultivars presenting a significant difference only for 10 mM nitrate and GLN (Table 4). This demonstrates that there were differences between the biomass partitioning in leaves and roots when different sources and doses of N were used.
The soluble protein contents of the leaves did not demonstrate a direct correlation with increased levels of N in the cv. BRS Planalto being that the increase in the nitrate availability had as a response decrease in soluble protein content probably due to the effect of high concentrations of nitrate (Figure 1).
Concerning the source of N in form of GLN, there was no significant difference between the levels of 0.1 and 10 mM. This may have occurred to treat yourself to a source organic N, that is produced endogenously. Comparing the two nitrogen sources, there were differences between only at 0.1 mM nitrate compared to the same level of GLN in the cv. BRS Planalto (Figure 1). Meanwhile, in the cv. BR5202 Pampa, the same behaviour was not observed since the dose of 0.1 mM nitrate did not differ from GLN (Figure 1). For cv. BR5202 Pampa, there was no difference in the treatments with nitrate and a significant difference was observed only for 10 mM GLN compared to 0.1mM and the control (Figure 1).
In the roots of cv. BRS Planalto, we observed higher protein content at 0.1 mM nitrate in relation to 10 mM. With GLN, there were no significant differences between treatments (Figure 2). The cultivar cv. BR5202 Pampa presented a higher content of protein with increased doses of nitrate or GLN. Shankar and Srivastava (1998) obtained an increment in the protein content in roots of corn seedlings subjected to Hoagland solution with 5 mM nitrate and after 1 and 5 mM of GLN.
The increase in protein content most likely occurs due to the incorporation of N of GLN in the other amino acids and eventually in proteins because in the roots, high concentrations of amides cause toxic effects in many processes related to protein metabolism.
In the comparison between cultivars, the two sources were similar for treatments with 0.1 mM compared with 10 mM, where plants cultivated with GLN accumulated higher contents of soluble protein in the roots.
In the leaves of cv. BRS Planalto, NR activity was decreased with increased doses of both sources (Figure 3). The cv. BR5202 Pampa also presented a decrease in activity of NR with increased dose of either source. These data are confirmed by the work of Oliveira et al. (2013) who tested the efficiency and use of N and enzyme activity in maize genotypes where it was concluded that the higher the concentration of NR, the lower the efficiency of N and its compounds in the plant.
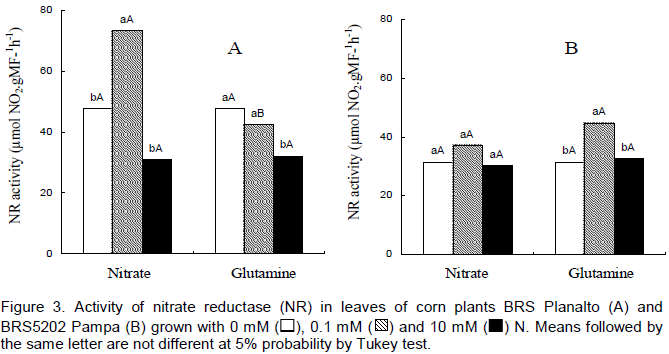
The behaviours were similar among cultivars with the exception of genotype BRS Planalto that presented the highest NR activity in relation to BR5202 Pampa in the treatment with 0.1 mM nitrate. The NR activity differed between blocks and N levels but did not show differences between maize genotypes utilized (Majerowicz et al., 2002). The NR activity was similar at 1.6 mM or 16 mM of N among 14 maize genotypes (Purcino et al., 1998). According to Miflin and Habash (2002), the action of glutamine and its enzyme is a key point for the growth and productivity of plants. For the cv. BRS Planalto, there was a decline in the foliar activity of NR when the level of nitrate increased from 0.1 to 10 mM (Figure 4).
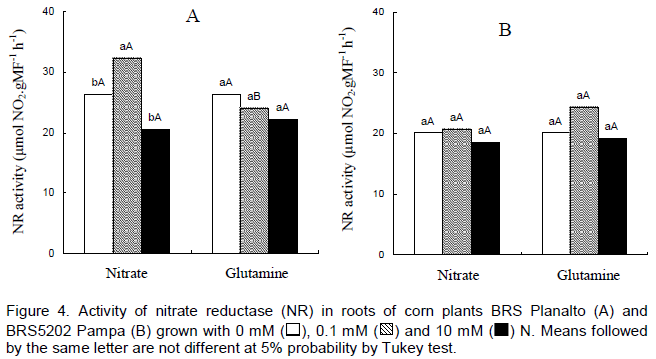
Despite that there were no significant differences; there was even a turn slightly to decrease the enzyme activity considering the GLN as a source of N both in the leaves as in roots (Figure 4). Silva et al. (2011), studying the effects of NR activity under fertilization of N and K reported that the higher the concentration of N, the smaller the NR enzyme activity. Shankar and Srivastava (1998) obtained a decline in the activity of NR with the increased concentrations of GLN in the roots of maize seedlings subjected to Hoagland solution with 5 mM KNO3 and thereafter to 1, 5, 10 and 20 mM of GLN. In contrast, Aslam et al. (2001) showed an increase in the NR activity in response to increased concentrations of GLN from 0.1 for 10 mM in barley plants.
The cv. BR5202 Pampa had a greater activity of NR in the roots compared with the BRS Planalto at the dose 0.1 mM nitrate. Normally, the enzyme NR does not present a high activity when evaluated in conditions of low levels of N, that is, N ≤ 300 kg/ha (Machado et al., 1992). In general, a decline of NR activity occurred in both genotypes in response to increased doses of N.
Purcino et al. (1998) evaluating seven of the cultivars utilized in his experiment presented a decline of NR activity with an increase of 1.6 to 16 mM nitrate. However, Majerowicz et al. (2002) obtained increased from 3.8 to 12.7 ± mol NO2- g-1 MF h-1 in the NR activity when the dose increased from 1 to 15 mM of N. The NR activity was always higher in the leaves in relation to the roots in both cultivars.
According to Shankar and Srivastava (1998), an increase in the protein content in corn seedlings (Z. mays L.) occurs after the addition of glutamine. However, Li et al. (1995) claimed that corn sprouts (Z. mays L.) subjected to glutamine demonstrated little or no effect on the activity of the NR.
The enzymes naturally presents a linear initial response as a function of substrate availability, then present a saturation to reach their maximum capacity of converting the substrate into their respective products, so it is of no use to increase the concentration of substrate when the enzyme has reached its maximum capacity of metabolism. In the specific case of NR, the form of nitrogen available in the middle is one of the factors that influence its activity. The nitrate is an inducer of enzyme activity, once an enzyme induced by substrate and not constitutive of the middle. However, in higher concentrations and depending on the age of the plant, this may not be beneficial and can cause a decrease in the activity of the enzyme as nitrite and ammonium chloride. Camargos, (2007) suggested that the action of the substrate and enzyme of NR is one of the greatest substrate-induced enzymes in higher plants.
In general, there was no significant difference between the solutions of N utilized. Likewise, Purcino et al. (1998) observed similarities among 14 corn genotypes in NR activity which was similar at 1.6 or 16 mM N. A large number of genes that work together or individually depending on the carbon and nitrogen available (Scheible et al., 1997) command the efficiency of nitrogen use. For the increased availability of nitrogen, a decrease in protein content can be occurred by excess resulting in accumulation of toxic forms of nitrogen and causing inhibition of enzymes involved in nitrogen metabolism. This may explain the fact of cultivars, possibly have different answers, the availability of nitrogen in the soil and by extension in the own plant. In this way, it is possible to find variation phenotypic and genetic in order to understand the genetic basis of efficiency of the use of nitrogen as a key component to the productivity of corn (Hirel et al., 2001).
The content of photosynthetic pigments showed no differences between the analysed genotypes, only between the doses tested. The soluble protein content was higher in leaves of BRS Planalto. The activity of nitrate reductase was greater at dose than 0.1 mM independent of the nitrogen source and organ involved. The activity of nitrate reductase is higher in leaves compared with roots, and glutamine does not inhibit its activity. Further studies are needed with other dosages and cultivars to clarify the action of these sources for the culture of corn and utilize such sources in practice in the camp.
The authors have not declared any conflict of interest.
REFERENCES
Alfoldi Z, Pinter L, Feil B (1992). Accumulation and partitioning of biomass and soluble carbohydrates in maize seedlings as affected by source of nitrogen, nitrogen concentration and cultivar. J. Plant Nut. 15:2567-2583.
Crossref |
|
|
Arnon DJ (1949). Cooper enzymes in isolated chloroplast: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24:1-15.
Crossref |
|
|
Aslam M, Travis RL, Rains DW (2001). Differential effect of amino acids on nitrate uptake and reduction systems in barley roots. Plant Sci. 160:219-228.
Crossref |
|
|
Britto DT, Kronzucker HJ (2002). NH4+ toxicity in higher plants: a critical review. Plant Physiol. 159:567-584.
Crossref |
|
|
|
Camargos LS (2007). Alterações no metabolismo de compostos nitrogenados em calopogonium mucunoides em resposta a diferentes fontes de nitrogênio: efeitos na nodulação e na fixação. 142f. [Tese] - Curso de Pós-graduação Biologia Vegetal, Universidade Estadual de Campinas, São Paulo. |
|
|
|
Castro MVL de, Naves MMV, Oliveira JP de, Froes L de O (2009). Rendimento industrial e composição química de milho de alta qualidade proteica em relação a híbridos comerciais. Rev. Pesquisa Agropec. Trop. 39:233-242. |
|
|
Cataldo DA, Haroon M, Schradev LE, Youngs VL (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Sci. Plant Anal. 6:71-80.
Crossref |
|
|
Clegg KM (1956). The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 7:40-44.
Crossref |
|
|
|
Costa EM (1986). Efeitos do aluminio, nitrato e amônio sobre a nutrição nitrogenada em Eucaliptus grandis Hill (Maiden). Viçosa: UFV, P. 50. |
|
|
Figueiredo CC, Resck DVS, Gomes AC, Urquiaga S (2005). Sistemas de manejo na absorção de nitrogênio pelo milho em um Latossolo Vermelho no Cerrado. Rev. Pesquisa Agropec. Bras. 40:279-287.
Crossref |
|
|
|
|
|
Hirel B, Bertin P, Quilleré I, Bourdoncle W, Attagnant C, Dellay C, Gouy A, Cadiou S, Retailliau C, Falque M, Gallais A (2001). Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 125:1258-1270.
Hirel B, Le Gouis J, Ney B, Gallais A (2007). The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exper. Bot. 58(9):2369-2387.
|
|
|
Kenis JD, Silvente ST, Luna CM, Campbell WH. (1992). Induction of nitrate in detached corn leaves: the effect of the age of the leaves. Physiol. Plantarum 85:49-56.
Crossref |
|
|
|
Lara Cabezas WAR, Arruda MR, Cantarella H, Pauletti V, Trivelin PCO, Bendassolli JA. (2005). Imobilização de nitrogênio da uréia e do sulfato de amônio aplicado em pré-semeadura ou cobertura na cultura do milho no sistema plantio direto. Revista Brasileira de Ciência do Solo 29:215-226. |
|
|
Li XZ, Larson DE, Glibetic M, Oaks A (1995). Effect of glutamine on the induction of nitrate reductase. Physiol. Plantarum 93:740-744.
Crossref |
|
|
|
Li XZ, Oaks A (1994). Induction and turnover of nitrate reductase in Zea mays L. influence of light. Plant Physiol. 106:1145-1149. |
|
|
Lichtenthaler HK (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 148:350-382.
Crossref |
|
|
|
Lillo C (2004). Light regulation of nitrate uptake, assimilation and metabolism. In: Amancio S, Stulen I. (Eds.). Plant Ecophysiol. Nitrogen Acquisition Assimilation Higher Plants 3:149-184. |
|
|
|
Machado AT, Magalhães JR, Magnavaca R, Silva MR (1992). Determinação da atividade de enzimas envolvidas no metabolismo do nitrogênio em diferentes genótipos de milho. Revista Brasileira de Fisiologia Vegetal 4:45-47. |
|
|
|
Majerowicz N, Pereira JMS, Medici LO, Bison O, Pereira MB, Santos Jr UM (2002). Estudo da eficiência de uso do nitrogênio em variedades locais e melhoradas de milho. Revista Brasileira de Botânica 25:129-136. |
|
|
|
Marenco RA, Lopes NF (2009). Fisiologia vegetal: fotossíntese, respiração, relações hídricas e nutrição mineral. 3.ed. Viçosa: UFV, 2009. P. 486. |
|
|
Mc Cready RM, Guggolz J, Wens HS (1950). Determination of starch and amylase in vegetables. Anal. Chem. 22:1156-1158.
Crossref |
|
|
|
Medici LO (2003). Cruzamentos dialélicos entre linhas de milho contrastantes no uso de nitrogênio. [Tese]. Piracicaba: Universidade de São Paulo, P. 88. |
|
|
Miflin BJ, Habash DZ (2002). The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exper. Bot. 53:370, 979-987.
Crossref |
|
|
|
Nelson N (1944). A photometric adaptation of the Somogy method for the determination of glucose. J. Biol. Chem, 153:375-380. |
|
|
|
Oliveira LR de, Miranda GV, Lima RO de, Fritsche-Neto R, Galvão JCC (2013). Nitrogen uptake and utilization efficiency and enzymatic activity in maize genotypes. Revista Ciência Agronômica, Fortaleza, 44(3):614-621. |
|
|
Pate TS (1980). Transport and partitioning of nitrogenous solutes. Ann. Rev. Plant Physiol. 31:313-340.
Crossref |
|
|
|
Purcino AAC, Arellano C, Athwal GS, Huber SC. (1998). Nitrate effect on carbon and nitrogen assimilating enzymes of maize hybrids representing seven eras of breeding. Maydica 43:83-94. |
|
|
Scheible WR, González-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M (1997). Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 9:783-798
Crossref
|
|
|
|
|
Shankar N, Srivastava HS (1998). Effect of glutamine supply on nitrate reductase isoforms in maize seedlings. Phytochemistry 47:701-706.
Crossref |
|
|
|
Silva EC, Buzetti S, Guimarães GL, Lazarini E, Sá ME de (2005). Doses e épocas de aplicação de nitrogênio na cultura do milho em Silva FAS (1996). The ASSISTAT Software: statistical assistance. In: International Conference on Computers in Agriculture, 6, Cancun, 1996. Anais. Cancun: American Society of Agricultural Engineers pp. 294-298. |
|
|
|
|
|
Silva FAS, Azevedo CAV (2002). de. Versão do programa computacional Assistat para o sistema operacional Windows. Revista Brasileira de Produtos Agroindustriais, Campina Grande 4(1):71-78. |
|
|
|
Silva FAS, Azevedo CAV (2006). A New Version of The Assistat-Statistical Assistance Software. In: World Congres on Computers in Agriculture, 4, Orlando-FL-USA: Anais. Orlando: American Society of Agricultural and Biological Engineers pp. 393-396. |
|
|
|
Silva FAS, Azevedo CAV (2009). Principal Components Analysis in the Software Assistat-Statistical Attendance. In: World Congress on Computers in Agriculture, 7, Reno-NV-USA: American Society of Agricultural and Biological Engineers. |
|
|
Silva SM da, Oliveira LJ, Faria FP, Reis EF dos, Carneiro, MAC; Silva, SM da. (2011). Atividade da enzima nitrato redutase em milho cultivado sob diferentes níveis de adubação nitrogenada e potássica. Ciênc. Rural Santa Maria 41(11):1931-1937.
Crossref |
|
|
|
Somogy M (1952). Notes on sugar determination. J. Biol. Chem. 95:19-23. |
|
|
|
Taiz L, Zeiger E (2009). Fisiologia vegetal. 4. Ed. Porto Alegre: Artmed, P. 848. |
|
|
Touraine B, Vedele FD, Forde BG (2001). Nitrate uptake and its regulation. In: Lea PJ, Morot-Gaudry JF. (Eds.). Plant Nitrogen. pp. 1-36.
Crossref |
|
|
Unno H, Uchida T, Sugawara H, Kurisu G, Sugiyama T, Yamaya T, Sakakibara H, Hase T, Kusunoki M (2006). Atomic Structure of Plant Glutamine Synthetase. J. Biol. Chem. 281:29287-29296.
Crossref |