ABSTRACT
The delay of maize harvest may affect the stem and grain sanity. This work was carried out aiming to evaluate the effects of harvest time on the incidence of stem rots and rotten grains of maize hybrids with contrasting growth cycles. The experiment was set in Lages, SC, during the 2012/2013 and 2013/2014 growing seasons. A randomized block design, disposed in split plots was used. Five single-cross hybrids were tested in the main plots: P1630H and P32R22H (hipper early cycle), P2530 (super early cycle) and P30F53YH and P30R50YH (early cycle). Five harvest times were assessed in the split plots: 0 (grain physiological maturity), 10, 20, 30 and 40 days after physiological maturity. The incidence of stem rots increased proportionally to the delay in harvest time, regardless of hybrid growth cycle. More than 60% of the stems presented rot symptoms when harvest was performed 30 and 40 days after physiological maturity. Such behavior enhanced the percentage of lodged and broken stems when harvest was postponed. Harvest time did not affect the percentage of rotten grains, which was higher for hybrid P32R22H due to its poor ear husk coverage. Harvest delay affected more significantly the stem than grain sanity of maize hybrids.
Key words: Zea mays, stalk rots, rotten grains, harvest delay.
The concomitant presence of maize and soybean is common in Southern Brazilian farms due to the need of establishing a crop rotation system. This management strategy is important to stimulate nutrient recycling, to increase soil water storage capacity, to enhance weed control efficiency and to prevent disease occurrence in both crops (Olibone et al., 2010; Castro et al., 2011; Franchini et al., 2012). The development of earlier ripening soybean cultivars and the anticipation of its planting date to early Spring (beginning of October) have accelerated soybean harvest to February, a month where maize is also ready to be harvested (Stülp et al., 2009). When this situation occurs, growers harvest soybean first because it is more profitable and more sensitive to harvest delay (Cella et al., 2014). Many times, such decision forces maize to stay in the field for over 30 days after grain physiological maturity (Panison et al., 2016).
The delay on maize harvest is a risky management decision because it brings several negative consequences, such as stem lodging and breaking, kernel germination at the ear and insect attack (Panison et al., 2016). These effects are more intense when maize is grown after plants of the same botanical family, such as black oat (Avena sativa). This species hosts fungi such as Fusarium graminearum and Colletotrichum gramínicola that cause stem and grain rot (Casa et al., 2007, 2009).
The intensity of stem lodging and breaking due to harvest delay depends on the hybrid’s traits, management practices (fertilization level, plant density and row spacing), meteorological conditions at the end of the crop cycle and damages caused by insects and diseases (Gomes et al., 2010). Soils with high fertility, crowded stands, windy and rainy conditions during grain filling increase the percentage of broken stems at harvest (Casa et al., 2007; Schmitt, 2014). Stem lodging is also enhanced because maize stores more than 50% of its biomass in the grain at harvest (Sangoi et al., 2010). Therefore, the longer maize remains in the field after grain physiological maturity, the higher is the risk of having broken stems before harvesting the crop (Ferreira et al., 2012).
The maintenance of maize kernels in the field for long periods of time after physiological maturity favors ear infection by fungi that decrease grain quality (Kaaya et al., 2005; Lauren et al., 2007). Harvest delay is the major responsible for the increase in rotten grains and mycotoxin content derived from the presence of Aspergillus, Penicillium and Fusarium (Marques et al., 2009). Such fungi use endosperm storage compounds as energy source for their growth and development (Alakonya et al., 2008). Therefore, they reduce kernel mass, changing grain quality and visual appearance (Pinto et al., 2007). Losses due to fungi attack usually range from 7 to 15%, but they can exceed 50% under extreme conditions (Kaaya et al., 2005). Furthermore, these fungi produce toxic chemical compounds, compromising the use of maize kernels to produce oil or feed humans and livestock (Zain, 2011).
The cultivar’s choice is a management strategy that can help to mitigate damages caused by late harvests. Maize hybrids present great variability regarding growth cycle duration, kernel type and plant resistance to diseases (Ferreira, 2012). The presence of decumbent ears at the end of grain filling maybe a desirable trait because it prevents water accumulation at the ear tip (Fonseca, 2005). Another favorable feature is adequate ear coverage with well developed husks. This trait avoids the exposition of kernels located at the upper part of the ear to unfavorable weather conditions (Guiscem et al., 2002).
This work was carried out based on two hypotheses: Harvest delay after grain physiological maturity increases the incidence of rotten stems, favoring plant lodging and decreasing kernel quality; the magnitude of damages caused by late harvest depends on the hybrid’s traits. The experiment aimed to evaluate the effects of harvest time on stem and kernel sanity of maize hybrids with different growth cycles.
The experiment was conducted in the city of Lages, Santa Catarina State, in the highlands of southern Brazil, during the growing seasons of 2012/2013 and 2013/2014. The experimental site is located at 27º52’ latitude south, 50º18’ longitude west and 900 m above sea level. The climate of the region is classified by Köppen-Geiger, mentioned by Kotteck (2006), as Cfb, presenting mild summers, cold winters and adequate rainfall during the whole year.
The soil at the study site was an Oxisol (Hapludox), according to Embrapa (2006), having the following chemical characteristics: Clay content, 560 g kg-1; organic matter content, 60.0 g kg-1; water pH, 5.2; SMP pH, 5.7; phosphorus, 4.4 mg dm-³; potassium, 186 mg dm-³; calcium, 5.79 cmolc dm-³; magnesium, 2.47 cmolc dm-³; aluminium, 0.2 cmolc dm-³; CTC, 8.94 cmolc dm-³.
A randomized block design arranged in split plots was used, with four replicates per treatment. Five single-cross hybrids with contrasting growth cycles were assessed in the main plots: Two hipper early hybrids (P32R22H and P1630H) that require 1282 and 1220 heat units (HU) to reach physiological maturity; one super early hybrid (P2530) that requires 1390 HU to attain physiological maturity; and two early hybrids (P30R50YH and P30F53YH) that achieve physiological maturity with 1493 and 1556 HU. Five harvest times were evaluated in the split plots: 0 (grain physiological maturity), 10, 20, 30 and 40 days after physiological maturity. The first harvest time for each hybrid was carried out when there was a visible black layer in the grain insertion point on the cob (R6 stage of the growth scale proposed by Ritchie et al. (1993). Each split plot comprised four rows, 0.7 m apart and 7 m long. All measurements were taken from the two central rows, leaving borders of 0.5 m at the end of each row.
The experiment was set using a no-tillage system under a dead coverage of black oat (Avena strigosa). The experimental area was planted with maize for three consecutive years before installing the trial. The soil fertilization was determined aiming to achieve a grain yield of 18,000 kg ha-1. Fertilization was performed at the sowing date by applying 30 kg ha-1 of N, 295 kg ha-1 of P2O5 and 170 kg ha-1 of K2O. The fertilizers were superficially placed close to the sowing rows. Nitrogen was also side-dressed, applying 250 kg of N divided into three equal parts when the plants were at the V4, V8 and V12 growth stages, according to Ritchie et al. (1993). Urea was used as the N source.
The experiment was hand planted on 12/5/2012 and 10/5/2013. The plots were over-sown, dropping three seeds per hill and thinned to the desired density (80,000 plants ha-1) when the plants had three expanded leaves.
The weeds were controlled with two herbicide applications. The first was carried out immediately after sowing and prior to plant emergence with a combination of atrasine (1,400 g a.i. per hectare) and metolachlor (2,100 g a.i. per hectare). The second application was performed when maize plants were at V4, using tembotriona (100 g ha-1 de i.a.). Army worm (Spodoptera frugiperda) was controlled by spraying the insecticides lufenuron + lambdacyhalothrin (15 + 7.5 g de i.a. ha-1) when the crop reached the V6 and V12 growth stages, according to Ritchie et al. (1993).
The percentage of lodged and broken stems was determined on the harvest day of each treatment. The plant that presents stem rupture below the ear insertion node was considered broken. The plant that has an angle between the lower stem inter-nodes and the soil smaller than 45º was considered lodged.
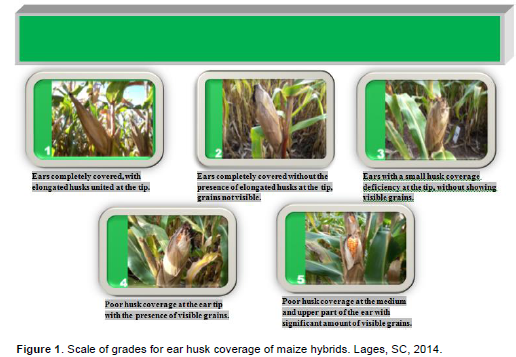
On the harvest day of each treatment, ear coverage by husks was also evaluated. A scale with grades ranging from 1 (best husk coverage) to 5 (worst husk coverage) was used to assess this variable. The evaluation was carried out visually, observing the extension of the modified leaves that protect the ear and identifying the presence of visible kernels not protected by husks at the ear tip. More details about the scale can be seen on Figure 1.
In 2012/2013, harvest time 0 (grain physiological maturity) was carried out on 05/01/2013 and 05/10/2013 for the hipper early and the other hybrids, respectively. In 2013/2014, harvest time 0 was performed on 03/06/2014, 03/16/2014 and 03/26/2014 for the hyper, super and early hybrids, respectively. The other harvests for each hybrid were performed at constant intervals of 10 days from harvest time 0. At all harvest times, the ears were collected manually.
The occurrence of stem rot was evaluated right after ears’ harvest. This evaluation was performed following the methodology presented by Reis and Casa (1996). The stems were cut with a knife nearly 30 cm above the soil surface. After that, they were longitudinally opened. The stems that presented internal visual discoloration symptoms were considered sick. The percentage of rotten stems was calculated dividing the number of stems with disease symptoms by the total number of stems of each split plot. A sample of symptomatic stems was taken to the Pathology Lab of Santa Catarina State University to identify the causal agents responsible for the diseases detected in the field.
At all harvest times, ears wee manually collected. Kernels were weighted and have their moisture contend determined. A kernel sample with 500 g was separated and placed in an oven at 65ºC until kernels were entirely dried. Subsequently, another sample of 200 g was taken to determine the incidence of rotten grains. Every kernel that presented more than 25% of its surface area discolored was considered rotten. The discolored kernels were weighted, allowing estimation of the percentage of rotten grains on each treatment.
The data were statistically evaluated by a variance analysis, using the F test, at the significance level of 5%. The data were previously transformed before carrying out the variance analysis using the expression (x + 1)1/2. When the F values were significant, the values were compared using Tukey’s test. The effect of delaying harvest time was also assessed by polynomial regression analysis, testing the linear and quadratic models. Both evaluations were conducted at the significance level of 5%.
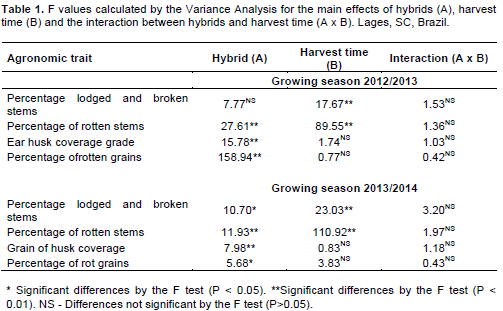
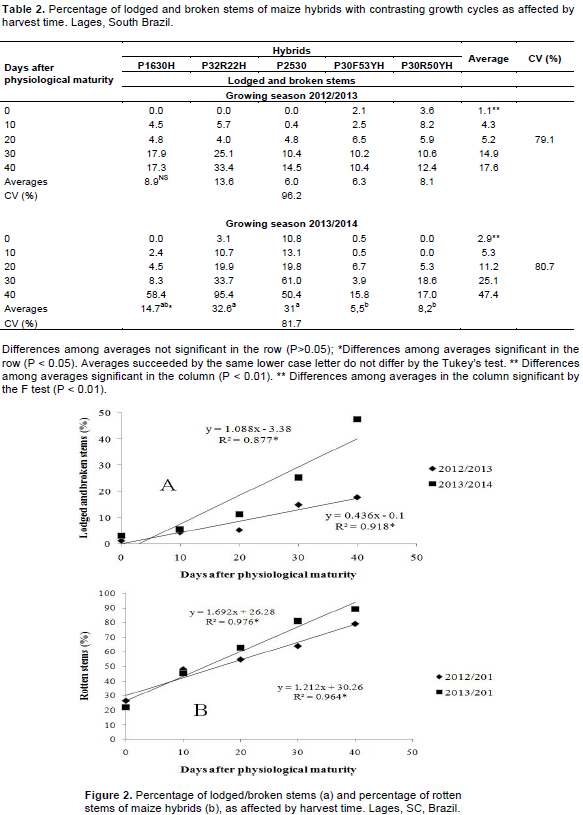
The percentage of lodged and broken stems was affected by harvest time at both growing seasons (Table 1). It ranged from 0 to 33.4% in 2012/2013 and from 0 to 95.4% in 2013/2014 (Table 2). In both growing seasons, the percentage of the lodged and broken plants increased proportionally to the delay in harvest time. The numeric values of the lodged and broken plants were greater in 2013/2014 than in 2012/2013. According to the regression analysis adjusted for the average values of the five hybrids, the percentage of lodged and broken stems presented a linear increase of 4.3 and 10.8% for each 10 days of delay in harvest time for the first and second growing seasons, respectively (Figure 2a).
In 2012/2013, there was no difference in the percentage of the lodged and broken stems among the hybrids (Table 2). Conversely, in 2013/2014 the hipper and super early hybrids P32R22H, P1630H and P2530 had a higher percentage of lodged and broken stems than the early hybrids P30F53YH and P30R50YH.
The delay of maize harvest time is a risky management strategy because it favours stem lodging due to wind and rain (Gomes et al., 2010). This tendency was confirmed in the present work for both growing seasons. Maize allocates more than 50% of the plant biomass to the grains at physiological maturity (Sangoi et al., 2010). Therefore, when the harvest is postponed, tissue senescence at the stem base and the constant presence of rainfall and temperatures below 17ºC that occur during April, May and June in the highlands of South Brazil (Table 3) increase the ear weight, favouring stem lodging and breaking. This behaviour is accentuated by the crop’s early ripening because maize hybrids with shorter growth cycles remobilize greater amounts of the stem-stored carbohydrates to the kernels during grain filling (Blum et al., 2003).
The percentage of rotten stems was affected by the main effects of hybrid and harvest time at both growing seasons (Table 1). The average value presented by the five hybrids for this variable was more than three times higher at the last harvest date than when maize was harvest at the grain physiological maturity (Table 4). The hipper and super early hybrids (P1630H, P32R22H and P2530) presented greater percentage of rotten stems than the early hybrids (P30F53YH and P30R50YH), at the average of harvest times According to the regression analysis, in 2012/2013 the percentage of rotten stems increased linearly 12.1% for each 10 days of delay in harvest time (Figure 2b). In the second growing season, the rate of increase in rotten stem incidence was 16.9% for each 10 days of harvest delay.
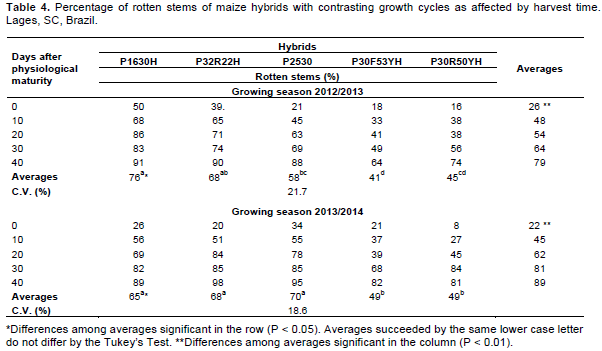
The rotten stems cause direct damage to maize due to the colonization of the vascular tissue, accelerating plant death (Romero Luna and Wise, 2015). Such behavior weakens the stem, favoring plant lodging and increasing grain losses during harvest (Casa et al., 2007). The greater incidence of rotten stems recorded in late harvests (Figure 2b) contributed to the higher values of stem lodging and breaking observed when harvest was performed 40 days after physiological maturity (Figure 2a). The cropping system used in the experimental area, with the succession of black oat and maize for three consecutive years, probably enhanced the percentage of rotten, lodged and broken stems when harvest was delayed. The three main fungi species detected in rotten stems were Colletotrichum graminicola, Stenocarpela macrospora and Fusarium graminearum, which are necotrophic pathogens favored by cropping systems that include plants of the same grass family (Casa et al., 2014).
The most efficient way to mitigate the negative effects of fungi infection to maize kernels is the correct hybrid choice (Carson et al., 2002). Cota et al. (2009) observed different responses of maize hybrids to the infection of C. graminicola, indicating that some genotypes presented higher ability to prevent the initial penetration of this pathogen inside the plant. Similar results were reported by Blum et al. (2003). These authors, testing cultivars with contrasting growth cycles, noticed that the hipper and super early ripening hybrids were more prone to rotten stems than the early hybrids. The same trend was detected in the present work. Hybrids with a short growth cycle also present small leaf area. Such trait increases the remobilization of carbohydrates from the stems to the kernels during grain filling, making the stems more susceptible to the infection of pathogens (Sangoi et al., 2010).
Harvest time did not interfere with the ear husk coverage grade probably because this trait is defined before the kernels achieve their physiological maturity (Tables 1 and 5). On the other hand, there were significant differences among hybrids regarding to this variable at both growing seasons. The hipper early hybrids P1630H e P32R22H showed the worst husk coverage, presenting exposed kernels at the ear tip (Figure 1). The fast cob expansion at the beginning of grain filling that characterizes early ripening maturing hybrids probably contributed to the poor husk coverage presented by P1630H and P32R22H (Guiscem et al., 2002).
There were significant differences among hybrids in the percentage of rotten grains at both growing seasons (Tables 1 and 6). The hyper early hybrid P32R22H presented the higher percentage of rotten grains, on the average of five harvest times. This behavior was probably caused by its worst ear husk coverage (Table 5). Poorly covered ears, with short and loose husks, are more susceptible to fungi infection, due to the easier access of fungi such as S. macrospora and F. graminearum to the kernels, which increase the occurrence of rotten grains (Costa et al., 2011).
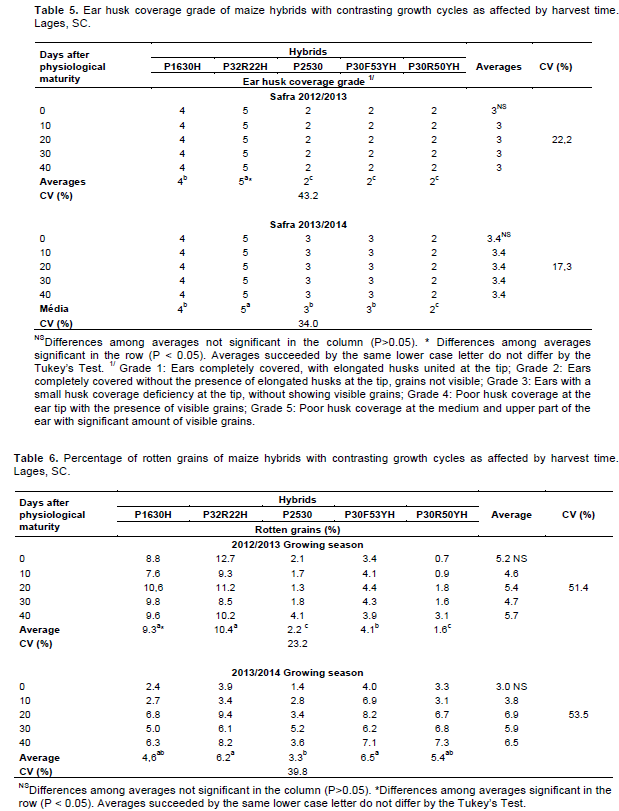
Harvest time did not affect the percentage of rotten grains (Tables 1 and 6). This result differs than the data reported by Santin et al. (2004) and Marques et al. (2009), who observed an increase in the amount of rotten grains when harvest was postponed. In the present work, harvest delay increased the percentage of rotten stems caused by S. macrospora and F. graminearum (Table 4). After infecting the stem, these fungi can migrate to the ear, enhancing the percentage of rotten grains (Casa et al., 2009). Nonetheless, there was no significant effect of harvest time in the percentage of rotten grains (Table 6). This apparent contradiction can be explained by the fact that the fungi that promote rotten ears and subsequently rotten grains infect the kernels during the early stages of grain filling. Therefore, they hardly colonize maize female inflorescence after the plant physiological maturity (Casa et al., 2014). Such behavior explains the lack of association between the increase in rotten stems and rotten grains incidence when harvest was delayed.
1. The delay of maize harvest time increased the percentage of rotten stems and the percentage of lodged and broken plants, regardless of hybrid’s growth cycle.
2. The delay of maize harvest time did not affect the percentage of rotten grains of the five evaluated hybrids.
3. The hipper early ripening hybrid P32R22 presented the worst ear husk coverage and the highest percentage of rotten grains, regardless of harvest time.
4. Harvest delay affected more significantly the stem than grain sanity of maize hybrids.
The authors have not declared any conflict of interests.
REFERENCES
|
Alakonya AE, Monda EO, Ajanga S (2008). Effect of Delayed Harvesting on Maize Ear Rot in Western Kenya. American-Eurasian J. Agric. Environ. Sci. 4:372-380.
|
|
|
|
Blum LEB, Sangoi L, Amarante CVT, Arioli CJ, Guimaraes LS (2003). Desfolha, população de plantas e precocidade do híbrido afetam a incidência e severidade de podridões de colmo. Ciênc. Rural 33:805-811.
Crossref
|
|
|
|
|
Carson ML, Goodman MM, Williamson SM (2002). Variation in aggressiveness among isolates of Cercospora from maize as a potential cause of genotype–environment inter-action in gray leaf spot trials. Plant Dis. 86:1089-1093.
Crossref
|
|
|
|
|
Casa RT, Morreira EN, Bogo A, Sangoi L (2007). Incidência de podridões de colmo, grãos ardidos e rendimento de grãos em híbridos de milho submetidos ao aumento na densidade de plantas. Summa Phytopathol. 33:353-357.
Crossref
|
|
|
|
|
Casa RT, Nerbass FR, Andriolli CF, Junior JALV, Reis EM, Sangoi (2014). Manejo de doenças da espiga e qualidade de grãos. In: Eficiência nas cadeias produtivas e o abastecimento global. Capítulo 13, Salvador, 21º ed. pp. 127-137.
|
|
|
|
|
Casa RT, Reis EM, Kuhnen JPR, Bolzan JM (2009). Controle de doenças de milho em sistema de plantio direto. Rev. Plantio Direto 112:15-21.
|
|
|
|
|
Castro GSA, Crusciol CAC, Negrisoli E, Perim L (2011). Sistemas de produção de grãos e incidência de plantas daninhas. Planta daninha 29:1001-1010.
Crossref
|
|
|
|
|
Cella V, Silva JF, De Azevedo PH (2014). Efeito da dessecação em estádios fenológicos antecipados na cultura da soja. Biosci. J. 30:1364-1370.
|
|
|
|
|
Costa RV, Cota LV, Cruz JC, Silva DD, Queiroz VAV, Guimarães LJM, Mendes MSM (2011). Boletim de Pesquisa e Desenvolvimento 38. Recomendações para a Redução da Incidência de Grãos Ardidos em Milho. Sete Lagoas - MG, Embrapa Milho e Sorgo.
View
|
|
|
|
|
Cota LV, Costa RV, Casela CR, Lanza FE (2009). Efeito da podridão de colmo, causada por Colletotrichum graminicola, na produção da cultura do milho. Circular Técnica nº 120, Sete Lagoas, MG.
View
|
|
|
|
|
Embrapa (2006). Centro Nacional de Pesquisa de Solos. Sistema brasileiro de classificação de solos. 2. ed. Brasília 306 p.
|
|
|
|
|
Ferreira C (2012). Recomendações para a Redução da Incidência de Grãos Ardidos em Milho. 80p. Dissertação (Mestrado). Universidade Estadual de Ponta Grossa, Ponta Grossa.
|
|
|
|
|
Fonseca MJO (2005). Sistemas de Produção Embrapa Milho e Sorgo. Sete Lagoas: Colheita e Pós colheita.
|
|
|
|
|
Franchini JC, Debiasi H, Balbinot Junior A, Tonon BC, Farias JRB, Oliveira MCN, Torres E (2012). Evolution of crop yields in different tillage and cropping systems over two decades in southern Brazil. Field Crops Res. 137:178-185.
Crossref
|
|
|
|
|
Gomes LS, Brandão AM, Brito CH, Moraes DF, Lopes MTG (2010). Resistência ao acamamento de plantas e ao quebramento do colmo em milho tropical. Pesqui. Agropecuária Bras. 45:140-145.
Crossref
|
|
|
|
|
Guiscem JM, Bicudo SJ, Nakagawa J, Zanotto MD, Sansigolo C, Zucarelli C (2002). Características morfológicas e fisiológicas do milho que influenciam a perda de água do grão. Rev. Bras. Milho Sorgo 1:28-37.
Crossref
|
|
|
|
|
Kaaya AN, Warren HL, Kyamanywa S, Kyamuhangire W (2005) The effect of delayed harvest on moisture content, insect damage, moulds and aflatoxin contamination of maize in Mayuge district of Uganda. J. Sci. Food Agric. 85:2595-2599.
Crossref
|
|
|
|
|
Kotteck M (2006). World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15:259-263.
Crossref
|
|
|
|
|
Lauren DR, Smith WA, Di Menna M (2007). Influence of harvest date and hybrid on the mycotoxin content of maize (Zea mays) grain grown in New Zealand. New Zeland J. Crop Hortic. Sci. 35:331-340.
Crossref
|
|
|
|
|
Marques OJ, Vidigal FPS, Dalpasquale VA, Scapim CA, Princinoto LF, Machisnski JM (2009). Incidência fúngica e contaminações por micotoxinas em grãos de híbridos comerciais de milho em função da umidade de colheita. Acta Scientiarum. Agronomy 31:667-675.
Crossref
|
|
|
|
|
Olibone D, Encide-Olibone AP, Rosolem A (2010). Least limiting water range and crop yields as affected by crop rotation. Soil use Manag. 26:485-493.
Crossref
|
|
|
|
|
Panison F, Sangoi L, Kolling DF, Coelho CMM, Durli MM (2016). Harvest time and agronomic performance of maize hybrids with contrasting growth cycles. Acta Sci. Agron. 38:2.
Crossref
|
|
|
|
|
Pinto NFJ A, Vargas EA, Preis RA (2007). Qualidade sanitária e produção de fumonisina B1 em grãos de milho na fase de précolheita. Summa Phytopathol. 33:304-306.
Crossref
|
|
|
|
|
Reis EM, Casa RT (1996). Manual de identificação e controle de doenças do milho. Passo Fundo: Aldeia Norte 80 p.
|
|
|
|
|
Ritchie R, Hanway JJ, Benson GO (1993). How a corn plant develops. Ames: Iowa State University Sci. Technol. 26 p.
|
|
|
|
|
Romero LMP, Wise KA (2015). Timing and efficacy of fungicide applications for Diplodia ear rot management in corn. Plant Health Progress
Crossref
|
|
|
|
|
Sangoi L, Silva PRF, Argenta G, Rambo L (2010). Ecofisiologia da cultura do milho para altos rendimentos. Lages: Graphel 84 p.
|
|
|
|
|
Santin JA, Reis EM, Matsumura ATS (2004). Efeito do retardamento da colheita de milho na incidência de grãos ardidos e de fungos patogênicos. Rev. Bras. Milho Sorgo 3:182-192.
Crossref
|
|
|
|
|
Schmitt A (2014) Arranjo de plantas para maximizar o desempenho agronômico de milho em ambientes de alto manejo. Tese (Doutorado). Universidade do Estado de Santa Catarina, Lages 226 p.
|
|
|
|
|
Stülp M, Braccini AL, Albrecht LP, Ávila MR, Scapim CA (2009). Desempenho agronômico de três cultivares de soja em diferentes épocas de semeadura em duas safras. Rev. Ciênc. Agrotecnol. 33:1240-1248.
Crossref
|
|
|
|
|
Zain ME (2011). Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 15:129-144.
Crossref
|
|