ABSTRACT
The mycotoxin contamination in foods has become a growing threat and aroused many researches in science area. According to the food control authorities, Ochratoxin A (OTA) is among mycotoxins priority of food contaminants. The current work focuses on coffee cherries quality environment in Ivory Coast. The neglecting of good agricultural practices would lead to recurrent contamination of ivorian coffee in mycotoxinogen fungi and mycotoxin. We investigated and sampled during the post-harvest drying process of Robusta coffee bean, brought from Ivory Coast in 2008 and 2009. Morphological identification of total fungal flora and the determination of OTA producers of Aspergillus section Nigri have been performed. The reliability of the overall evaluated fungal contamination is estimated at 97%, being 7.03 in one coffee sample package of 300 g. Strains were isolated on potato dextrose agar (PDA) by the direct plating technique, and were grown at 25°C. Morphological study was performed using macroscopic and microscopic morphological characters. From the two hundred and eighteen strains of fungi isolated, the following were identified: Aspergillus section Nigri, Aspergillus section Fumigati, Penicillium, Mucor, Fusarium amongst others. Aspergillus section Nigri was found to be the most important group representing 52% of the population. Within this section OTA production was evaluated on czapeck yeast agar (CYA) and quantify by High-performance liquid chromatography (HPLC). Twenty percent of produced detectable OTA with concentrations ranging from 0.3 to 56 µg/g of agar medium. The objectives of this study is to define the risk contamination in post-harvest fungi on coffee.
Key words: Agriculture, coffee, process, filamentous fungi, mycelium, Aspergillus, mycotoxin, Ochratoxin A.
Coffee is very valuable. However, it is subjected to various pest and diseases of which mycotoxin contamination is of great importance in terms of the health of consumers and economic loss (Paterson et al., 2014). Fungal contamination of coffee has been reported from all over the world. Many researchers have worked on coffee microbiology process (Silva et al., 2000; Pandey et al., 2000; Noonim et al., 2008; Nakajima et al., 1997; FAO, 2006a) and show that microorganisms are naturally present in all pre-and post coffee harvests and might influence coffee quality. Microbial biodiversity present in coffee cherries and beans depends on the coffee variety, processing method, and environmental factors of the region in which they are cultivated (Bucheli and Tanikawa 2002; Batista et al., 2009). Two methods are carried out for the post-harvest process of coffee: dry and wet methods (Schwan and Wheals, 2003). Therefore the impact of microbial and their distribution vary from one treatment to another. From East to West area, post-harvest and cultural practices differ according to the income of farmers. Throughout coffee area in Côte d’Ivoire, post-harvest treatments have been improved. In the some localities where the farmers suffer the pangs of more tradition and are threatened constantly by poverty, post-harvest treatments are more rudimentary. Generally ripped cherries are harvested in one go and dried on traditional media. Then they are peeled by machine or pounded in a mortar and stored in bags of raffia, at the farmer or in storage. After shelling, coffee cherries undergo a process of natural drying on different support like rack, plastic film, ground, asphalt and cement. The type of drying is free and depends on the income of the farmer. Tropical moisture and storage conditions sometimes expose cherries and coffee beans at a constant postharvest mold contamination. Some studies on coffee (Kouadio et al., 2010, 2012), report that fungi are constantly present on coffee samples from Côte d’Ivoire, whatever their type and conditions of post-harvest treatment. Endogenous mycobiota of coffee cherries, coming from coffee cherries ecosystem and various contaminations which it is subjected during steps of washing cycle (more or less microorganisms in water), drying, transport and storage are also potential sources of contamination in filamentous fungi. Mycobiota involved in coffee fermentation or contamination come from the commensally flora of the cherries, soil, washing water and equipment used. The microbial load depends on physico-chemical parameters (temperature, pH, Aw), the composition of the substrate in simple sugars and/or polysaccharides, but also for the maintenance of equipment used, drying areas, storage and warehouses quality. Research reports that moisture pulp and its mucilaginous material (regarding its biochemical composition) promotes development of this endogenous mycobiota (Avallone, 2001).
Several mycotoxigenic strains of Aspergillus section Nigri have been isolated on Robusta coffee in Côte d'Ivoire (Kouadio et al., 2012), in Brazil (Taniwaki et al., 2003), in Thailand (Noonim et al. 2008; Joosten et al., 2001) and Vietnam (Leong et al., 2007).Within them Aspergillus spp. like Aspergillus carbonarius, Aspergillus niger, Aspergillus terreus in section Nigri and Aspergillus versicolor, Aspergillus ochraceus, Aspergillus alliaceus (Bayman et al., 2002) in other section have been recognized as Ochratoxin A producers in Wheat (Riba et al., 2008), in cocoa beans (Sanchez-Hervas et al., 2008), in robusta coffee beans (Kouadio et al., 2012), in peanuts seed (Magnoli et al., 2007), in grapes (Salma et al., 2007) and olive fruits (Roussos et al., 2006). Other strains as Aspergillus sclerotioniger and Aspergillus lacticoffeatus are rarely OTA producing strains. Contamination of food commodities, including cereals and cereals products, nuts and species with OTA has been reported from all over the world. Mycotoxins have been ranked as ‘‘the most important chronic dietary risk factor, higher than synthetic contaminants, plant toxins, food additives, or pesticide residues’’ (Kuiper-Goodman, 1998; Bennet and Klich, 2003). In fact food contamination by mycotoxins has been recognized as a public health threat (JECFA, 2002). Ochratoxin A as well as the Aflatoxins B1and M1and the Deoxynivalenol (DON) have been classified in the four priority mycotoxins of food contaminants by the Global Environment Monitoring System/Food Contamination Monitoring Assessment Programme (GEMS/Food) of the WHO (2002). Ochratoxin A mycotoxin produced by fungi of the genera Aspergillus and Penicillium, is a food contaminant found naturally in various agricultural products such as grains, oil seeds, nuts, coffee and cocoa (EU, 2010; EFSA, 2006; CCA, 2007). OTA is a critical element in the quality of food because it is thermostable and can be found in the finished products from agricultural raw materials contaminated even after industrial processing well done. Indeed, this may be a great loss if a food highly contaminated with OTA is declared unsuited for human and/or animal consumption (Dano Djédjé et al., 2009). Chemically Ochratoxin A, belong to a group of fungal metabolites that have a wide variety of toxic effects. It has been listed as possibly carcinogenic to humans (group 2B) by the International Agency for Research on Cancer (IARC) (Castegnaro et al., 2006). It causes renal toxicity, nephropathy and immuno-suppressive in several animal species, resulting in reduced performance parameters in animal production. OTA has also been detected in blood and other animal tissues and in milk, and has been implicated in the fatal human diseases Balkan Endemic nephropathy (Marquardt and Frohlich, 1992). Since it has been discovered several analytical methods already formally validated in team-work such as qualitative and quantitative methods are available. The particularity of qualitative techniques as ELISA kits rapid detection or Immune Radio Essay (RIA) is the fact that they require little purification, whereas techniques of quantitative detection require analysis method including a more complex purification-extraction phase and an analytical procedure (Berger et al., 1999). For filamentous fungi, technique qualitative detection of Ochratoxin A consists in demonstrating toxin production after growth of filamentous fungi on a specific agar to 60% of coconut cream. The visualization of fungal colony under UV light shows a blue-violet fluorescence around the strain tested if it is Ochratoxin A producer. As for quantitative techniques, they are modelled on analytical methods accepted by chemists internationally (AOAC, 2000; Raquel Duarte et al., 2008). These methods are constantly being updated in line with technological progress (CCA, 2007). The process of studying the ochratoxinogen potential of filamentous fungi require an extraction step focus on various media: solid synthetic media (czapeck yeast agar) (Bragulat et al., 2001) or liquid media (yeast extract sucrose broth, potato dextrose broth) (Bayman et al., 2002), and natural carbon substrates such as rice according to the official FDA method (Tournas et al., 2001), the coffee bean (Pitt, 2000). It has followed by a purification step which using methods are based on the specific nature of the solvent for OTA and its ability to releasing all interfering compounds OTA. Analytical procedures for mycotoxins determination have been improved continuously over the past years. Chromatographic methods have been used widely, including thin-layer chromatography (TLC) (Pittet and Royer, 2002), gas chromatography (GC) with electron capture detection (ECD) or mass selective detection (MS) as well as high-performance liquid chromatography (HPLC) with UV or fluorescence detection, also with (multiple) mass spectrometry as described in more recent publications (Berger et al., 1999). It should be noticed that there is no preset method of analysis because the analytical procedures and purification extraction methods are multiple and closely linked to the nature of the extraction matrix of OTA and OTA material analysis (solvent extraction, purification kit, quantification and detection devices) which represent a significant financial cost. For certain foods such as coffee, wheat, rice, grapes and wine, some analysis procedures OTA strongly in force have been perfected on Coffee (Bandeira et al., 2008) grain cereal (Tournas et al., 2001), grapes and wine (EU, 2010). These studies represent a preliminary investigation to assess the factors affecting the coffee beans during the post-harvest treatment, to collect and identify post-harvest molds isolated from coffee and detect OTA producing strains.
Sampling of coffee beans collection
Surveys and sampling were undertaken during (the coffee year) coffee campaign 2008 and 2009. Sixteen varied samples are collected during survey in 2008: dried coffee cherry ground, green coffee beans in storage, coffee husks. Fifteen dried cherries were collected in 2009. The cherries present in all samples were hand-picked and have been processed by dried natural process, on different drying support: plastic film, ground, and cement. They were sampled in sterile plastic bags and stored at room temperature (30°C), then were brought to the research center.
Isolation and purification of fungi
Fungi analysis were carried out on coffee sample of 300 g each one. Each sample was sub-sampled by taking three coffee cherries per 300 g of sample. Isolation was performed by direct plating technique (Perrone et al., 2007), on PDA medium (potatoes extract 4 g/L, dextrose 20 g/L, agar 15 g/L, distilled water 1 L). This technique allows growing most of the fungi present on the coffee cherry. From each sample, three coffee cherries per 300 g of sample were picked at randomly and applied to the surface of an agar medium PDA. Coffee cherries were incubated at 25 and 45°C for at least three days, after which we could see on the cherries and beans surface many fungi colonies of various genus (Figure 1a, b). Once purified on PDA agar, representative strains of each colonies were stored on PDA agar slants at 4°C. Determination of fungi genus was made by studying the morphological analysis.
Morphological analysis for identification
The phenotypic analysis of the filamentous fungi need references strains from international collection provided and by the Museum of the natural history of France. Morphological analysis of different fungi genus was applied by macroscopic and microscopic description of vegetative form called “the thallus” which allowed to differentiate the mycelium and conidia. Macroscopic studies are undertaken by observing fungi colonies with the naked eye and binocular microscope, to distinguish the main features of the fungal thallus. The various criteria studied in the mycelium are the color, the texture, the setbacks of the thallus and the contour. The criteria considered for the spores are: powdery or granular, the color, the size, the density and the volatility. More apical speed of growth rate was measured to characterize the various fungal groups.
Microscopic study focused on the characteristics of the tubular filament of mycelium (septate or no septate), the shape of the conidial head (Aspergillus), the structure of the conidiophores (Penicillium), the presence of phialides and metulae at conidiogenous cells, and the size of conidia, their shape and external ornamentation (Botton et al., 1990; Samson et al., 2007). This stage is developed by, observing a piece of mycelium and conidial head, the objective (x40), between slide and cover slip in Bleu Cotton.
Determination of OTA-producing fungi by the method plug agar
OTA production and extraction from Aspergillus section Nigri isolates
Aspergillus section Nigri was investigated for OTA production. Strains were grown on CYA (Czapeck Yeast Agar) for 7 days at 25°C. Then OTA was extracted with methanol (Bragulat et al., 2001) following the methodology described by Sanchez-Hervas et al. (2008): three agar plugs of 5 mm diameter were removed of the central, the middle and the extremity of each colony, in triplicate. The agar plugs were ground in 900 µl of methanol. After 24 h of storage in the dark at 4°C, the extract was centrifuged at 1300 rpm (15 min) and filtered using a cellulose acetate filter brand Whatman (0.45 µm). The filtrate was stored at -20°C until HPLC analysis (Sanchez-Hervas et al., 2008).
OTA detection and quantification from Aspergillus section Nigri extract
OTA quantification was carried out with an HPLC system by spectrofluorimetry (Nakadjima et al., 1997) using a fluorescence detector (Agilent Technologies 1200 serial). The detection was performed at the following wavelengths, 335 nm for excitation (λ exc) and 460 nm for emission (λ em). The wavelengths are specific for the fluorescent molecule. The fluorescence intensity depends on the concentration of the molecule. The detector is provided with a guard column (Nomura chemical) C18 column (Atlantis, 5 µm, 4, 6 x 250 nm). The following solvents were used for OTA analysis: acetonitrile, methanol and water (Sigma Aldrich), and the acetic glacial Carlo Erba. OTA standard was provided from a commercial stock solution of Ochratoxin A, 100 ng/ml (Petromyces albertensis assay ≥98%, Sigma Aldrich). The mobile phase (water/acetonitrile/acetic acid, 57/41/2) was pumped at an isocratic flow of 1 ml/min. OTA was identified by its retention time at 9.7 min according to a standard. The repeatability of the analysis was checked by standard solutions of OTA concentration and quantification by comparison with a calibration curve. The detection limit was 0,025 ng/ml.
Fungi genus classification
Genus identified in this collection (
Aspergillus section
Nigri, the
Aspergillus section
Fumigati,
Penicillium,
Fusarium and
Mucor) are mesophilic because they have been isolated only at 25°C, except
Aspergillus section
Fumigati which have grown both 25 and 45°C. Macroscopic analysis of fungi aims to study the characteristics of the fungal thallus, such as the appearance of colonies, their relief and color. Microscopic examination focuses on structure of the mycelium (Cahagnier and Richard-Molard, 1998), the conidiophore (De Hoog and Guarro, 1995), conidiogen cells (De Hoog and Guarro, 1995) and conidiospores (Botton et al., 1990). The majority of
Aspergillus section
Nigri colonies on PDA at 25°C have a woolly, yellow or white mycelium, with a cream reverse color characterized by roughly tightened striations.
Aspergillus section
Nigri mycelium under optical microscope is septate. Following the color of spores and their dispersion on the thallus,
Nigri section was divided in two groups.
The[C1] thallus of the first group is white with long trunk and large spores scattered through the thallus. Conidial head is globular, biseriate and spores are large size and wall-warted (Figure 2a, b).
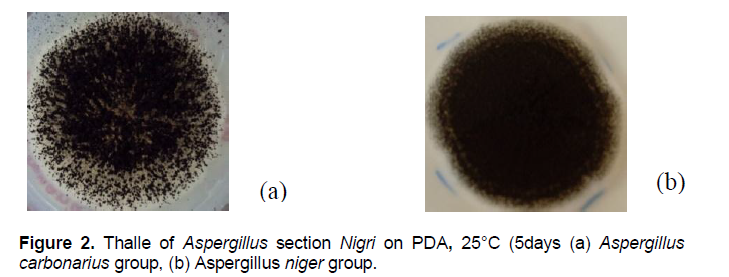
This first group might similar to Aspergillus carbonarius. The second group consists of other strains of Aspergillus section Nigri of which criteria are different from A. carbonarius characteristics, whose spores are dark brown to brown, tightened against the other, lining the thallus and giving it a dense and powdery appearance.Their stipe is invisible to the naked eye (Figure 3a, b).
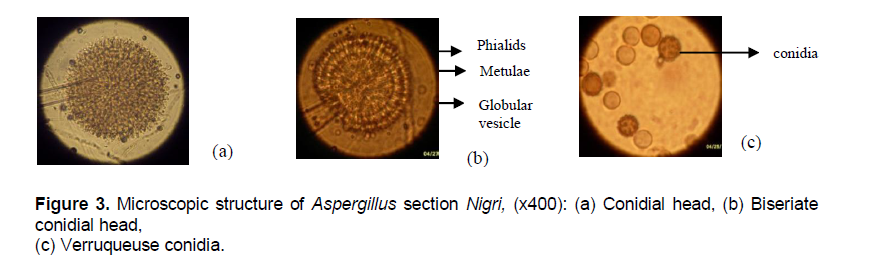
The second group is represented by the sub-group of the complex Aspergillus niger aggregate representing by A. niger, Aspergillus tubingensis, Aspergillus foetidus, Aspergillus piperis, Aspergillus brasiliensis, Aspergillus ibericus, Aspergillus costaricensis, Aspergillus uvarum, Aspergillus vadensis and Aspergillus lacticoffeatus (Samson et al., 2004).The conidia of the first group are wide spores warty-wall (Figure 3c) while in the second group they are mainly medium-sized and wall-echinulate (Albarca et al., 2004; Samson et al., 2007). Within the genus Aspergillus the comparison of the conidial head allowed to distinguish Aspergillus section Nigri which is globular type from Aspergillus section Fumigati which is clavatus type (Figure 4a, b). Following the mode of implementation of conidiogenous cells on the conidial heads, strains of Aspergillus section Nigri, were divided into two subgroups: the uniseriate and biseriate. Uniseriate strains count only one level of conidiogenous cells called phialides. On the other hand in biseriate strains, there are two levels of conidiogenous cells: metulae directly attached to the conidial head, carrying to their end phialides which are fruiting structures of conidia.

Colonies of Aspergillus section Fumigati have a fluffy appearance with a white mycelium, upholstered with green-dark spores (Figure 4a). The reverse is whitish and streaked. All strains of Aspergillus section Fumigati are uniseriate. Conidiogenous cells composed only of phialids cover a third party of conidial head which is claviform vesicle shape (Figure 4b). The mycelium is septate. They have the particularity to grow both at 25°C than at 45°C, making it mesophilic and thermophilic strains.
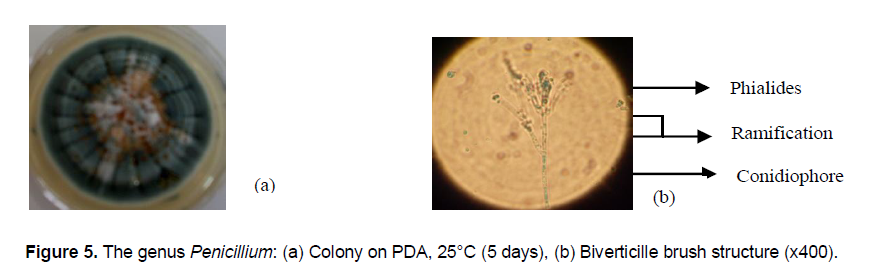
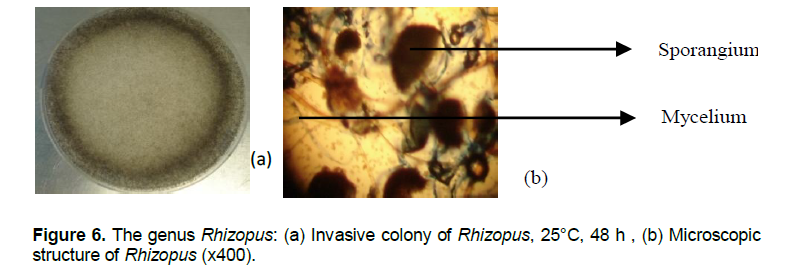
Fungi of the genus Penicillium have fluffy thallus covered by a powdery blue-green conidia (Figure 5a). The egg-shaped small conidia are hanging on a biverticille brush conidiophore with phialide hanging conidia (Figure 5b). Fusarium colony are pink fluffy. Microscopic structure shows egg-shaped conidia.The genus Rhizopus remarkably faster in growing whith an apical speed of growth rate which varies between 1.5 cm/day and 2 cm/day can get a diameter of 9 cm in 48 h and invade the Petri dish. Rhizopus colonies are very invasive on the surface of agar media (Figure 6a). Their thallus is white to grey and air-stringy with round-smooth-black structures called sporangium. Under optical microscope spores or sporangiospores are enclosed in the sporangium (Figure 6b).
Identification considered criteria (characteristics of the thallus, hyphae, conidiophores, conidiogenous cells, conidia) in this study are consistent with those defined by Botton et al. (1990) and Cahagnier and Richard-Molard (1998) and Hoog and Guarro (1995). Current taxonomic identification of filamentous fungi is based on micro-and macro morphological characteristics. The main identification markers in filamentous fungi are the cultural characteristics including colony on specific characteristics culture media (color, size, and shape), and development of sexual and asexual spore-forming structures, and / or physiological characteristics such as the ability to utilize various compounds as nitrogen and carbon sources (Glass and Donaldson, 1995; Cahagnier and Molard-Richard, 1998).
The main genera isolated and identified in this study are Penicillium, Aspergillus and Rhizopus. The genus Penicillium is characterized by an apical speed growth rate very slow ranging from 0.3 cm/day and 0.5 cm/day, which makes them difficult to transplant during purification. Whereas Aspergillus grow faster with an apical speed of growth rate ranging from 1.25 to 1.9 cm/day. Optical microscope Penicillium differ to Aspergillus by their brush structure and conidia of round or ovoid arranged chain shape. As for Aspergillus they are characterized by Aspergillus conidial head whose head struck form or claviform is uniseriate or biseriate (Botton et al., 1990; Clenny, 2005). Macroscopic study by comparison with reference strains was able to differentiate the strains related to Aspergillus carbonarius strains belonging to Aspergillus Niger aggregate group by the size of the spores. All strains isolated during this study and classified the species Aspergillus fumigatus, have the particularity to grow between 45 and 50°C, growth temperature at which A. fumigatus is different from other related species (Aspergillus fumigatiaffinis, Aspergillus novofumigatus, Aspergillus lentuli) capable of growth at 10°C.
Occurrence of fungi on coffee
Results of mycological study on coffee samples was estimated two hundred and eighteen strains isolated mold spreading across the two years of campaigning in the following proportions 53% in 2008 and 47% in 2009 (Table 1). Several types of mold at different rates have been identified, there are: Aspergillus section Nigri (53%), Aspergillus section Fumigati (13%), Penicillium (10%), Rhizomucor (16%), Fusarium (4%), Other (4%). This diversity is strongly represented in 2008 by five genera (Aspergillus section Nigri, Aspergillus section Fumigati, Penicillium, Rhizopus, Fusarium) and only three (Aspergillus section Nigri, Penicillium, Rhizopus) in 2009, one year to the next the flora largest collection of mold remains the Aspergillus section Nigri to 28% for 2008 and 82% to the account of 2009. The same genus were isolated from coffee samples from different origins by Pardo et al. (2004) with a predominance of Aspergillus section Nigri at a rate of 65.4%. Genus Aspergillus was the major contaminant samples of coffee every year that to say 33% in 2008 and 84% in 2009.
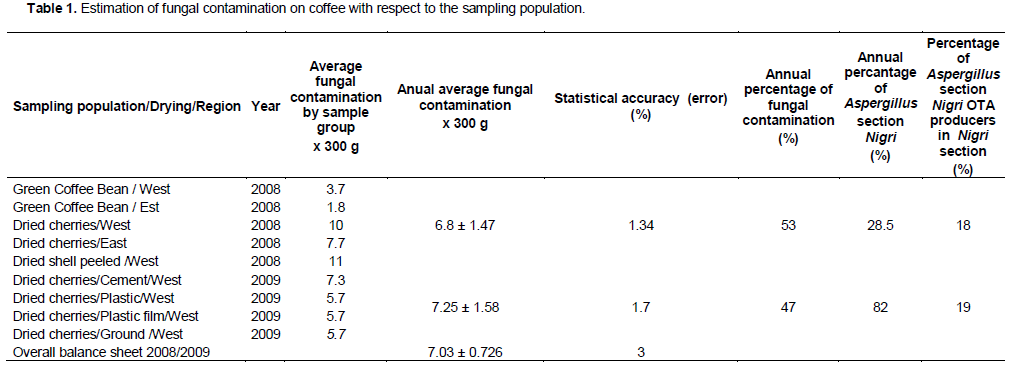
State of fungi contamination
Table 1 shows the state of fungal contamination over the entire sample population on coffee sample of 300 g each one sampled in 2008 and 2009. This population are very heterogeneous features more by the diversity of the types of samples, the sampling site (in 2008) and the variation of the drying yard (in 2009), than by their origin. Groups of samples are classified according to the type of samples (dried cherries, nuts, green beans) and types of post-harvest treatments or sampling sites (plantations, warehouse, shellers, drying area treatments, no-determined). The sampling was conducted according to a completely randomized to a repeat. One treatment (isolation of mold at 25°C on PDA agar by direct contact) has been carried out on one coffee sample batch of 300 g for qualitative post-harvest filamentous fungi research. Significance or accuracy of fungal contamination in relation to the sampling population considered was obtained by the inverse of the variance. The entire sample population is heterogeneous; there is loss of precision on the actual level of fungal contamination. In 2008, there are 98.6% chance that the average fungal contamination varies in a range from 6 to 9 fungi per batch of 300 g of coffee samples. Whereas in 2009 the level of fungal contamination estimated at between 5 to 8 fungi per batch of 300 g coffee samples is 98.3% reliable. The overall fungal contamination evaluated on campaigns in 2008 and 2009 is 97% reliable and is estimated at 7.03 of fungi in one coffee sample batch of 300 g.
There is no significant difference between the mean of fungal contamination in 2008 and 2009 as the mean difference (0.45) was less than the least significant difference (119.16). If the level of fungal contamination does not vary from one year to another it means that the factors of change that have occurred from one year to the other during the sampling season, sampling sites, the climate and the drying conditions (rain, prolonged sunshine...) did not influence fungal contamination. However, some groups of samples like dried cherries/west in 2008 and cement dried/cherries/west in 2009 are representative of the level of fungal contamination evaluated during these years. There is no correlation between the type of samples, post-harvest treatments and the level of fungal contamination. If the level of fungal contamination does not change, that means that the variation factors are environmental factors such as climate, varing little from east to west from one year to another.
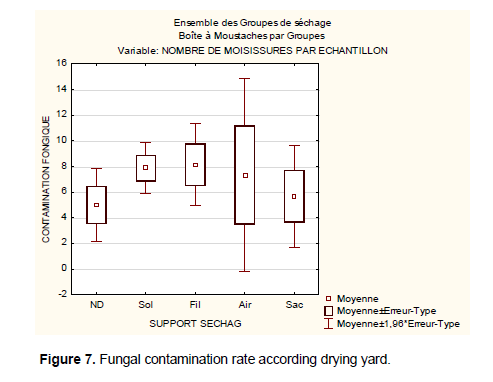
The results obtained from this evaluation can not allow to establish a correlation between the level of fungal contamination and different drying racks. Contrary to the recommendations of good agricultural practices all areas of drying without distinction have the same level of fungal contamination. This analysis is supported by (Figure 7) for studying trends by showing the variability of fungal contamination on different drying area: non-drying determined (ND), the drying ground (Sol), the drying cemented area (Air), drying on a plastic film (Fil). On non-determined supports the average rate of fungal contamination is 5 fungi per sample. It can vary within a confidence interval of 3.8 to 6.5 molds. The extent or limit of dispersion (if the parameters vary) varies between 2.1 and 7.8. It means that the average of fungal contamination in case of Good Agricultural Practices or implemented HACCP quality methods can fall to 2 fungi per samples of 300 g. In extreme cases of stress or slackening during good agricultural practices application (use of improved technology such as rack, the plastic film and maintenance, protection against moisture coffee, aerated storage drying ...) fungal rate contamination may progress to 7.8 per sample of 300 g. It is observed that there is an inconsistency between the means of contamination and post-harvest treatments during drying. Indeed considering the customs of the farmers in some villages healthy of drying areas is no longer a distinctive drying quality because other drying areas (the cemented area, the bag and the film plastic) trampled by farmers are subject to soil contaminants. The highest risk of exposure to contamination was characterized by fruit contact with the soil, constituted by the fraction coffee swept from ground, and by inadequate post-harvest handling of the product during drying in ground patios. Ground patios must be avoided, since soil is the natural habitat of ochratoxigenic fungi and other microorganisms as well-other (Batista et al., 2009).
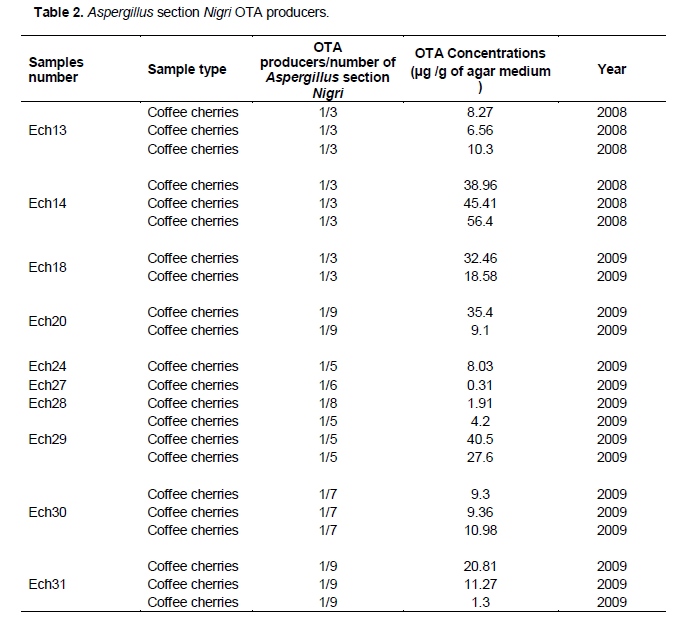
OTA profil of black Aspergillus section Nigri
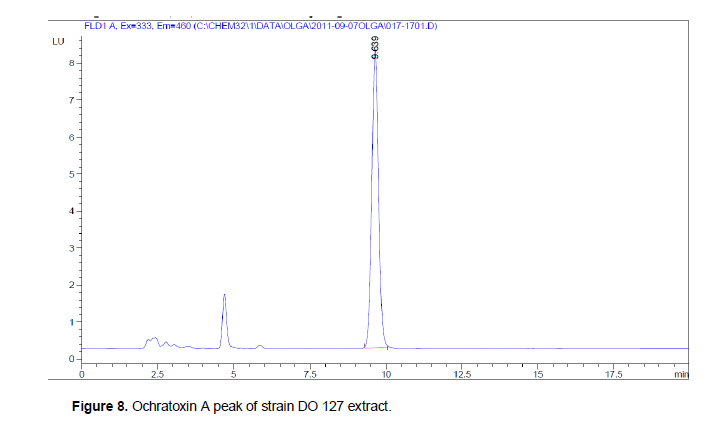
Mycotoxin analysis revealed that some strains of Aspergillus section Nigri isolated on robusta coffee in Côte d'Ivoire are able to produce OTA (Table 2). OTA production is brought out by a pic at a retention time of 9,639 (Figure 8). From all strains evaluated during 2008 and 2009 seasons, 11% of OTA producers were detected with levels ranging from 0.3 to 56 µg/g of czapeck yeast extract agar medium. The isolates considered as OTA producers presented a retention time similar to that of OTA standard. It is common to find in Aspergillus section Nigri producing Ochratoxin A.The results of the most sophisticated chromatographic procedures depend on the efficiency of the prior sample preparation, in particular on sampling, extraction and the further treatment of the extract, including any purification. As a large number of interfering compounds present in samples may contaminate the primary sample extract, these components must be removed as completely as possible for most method applications (Krska, 1998). The study of the production potential of OTA shows that strains do not possess the same level of concentration of Ochratoxin A. Some strains of the same sample, levels of OTA concentrations are sometimes close to each other (sample 30) or very distant (sample 29).
In coffee contamination by mold Aspergillus including A. carbonarius and A. niger, has been reported in several
countries (Perrone et al., 2007). Aspergillus are responsible for many diseases in plants and agricultural products from harvest to process transformation via by post-harvest treatments (Perrone et al., 2007). In studies conducted in Brazil it is mainly Aspergillus section Nigri that are contaminants of coffee and individuals Aspergillus niger (83.3%) (Martins et al., 2003) and A. carbonarius (Magnani et al., 2005). Similarly Pardo et al. (2004) have isolated a high percentage of Aspergillus section Nigri (65.4%) on coffee samples. In Vietnam, Leong et al. (2007) were isolated from coffee cherries robusta and arabica, strains of A. carbonarius. Results with the Aspergillus section Nigri were published as dominant for samples of arabica coffee beans collected in Brazil (Batista et al., 2009). According to Albarca et al. (2004), many species within Aspergillus section Nigri are OTA producers, mainly A. niger and A. carbonarius. Reported results (Table 2) on OTA production vary between 0.3 to 56 µg/g. It similar to others as Riba et al. (2008) (0.01 to 0.17 µg/g) and Sanchez-Hervas et al. (2008) (0.2 to 90 µg/g), who used the same method (Filtenborg and Frisvad, 1980; Batista et al., 2003) to extract OTA from filamentous fungi isolated on coffee and cocoa beans. Ochratoxin A is one of the most dangerous mycotoxins produced by certain important filamentous fungi such as A. ochraceus (Van Der Merve et al., 1965), A. carbonarius (Teren et al., 1996), with some isolates of A. niger (Abarca et al., 2001) and Penicillium verrucosum (Schmidt-Heydt et al., 2010). In case of multianalyzes screening an immunochemical biosensor assay for the detection of multiple mycotoxins (Aflatoxin B1, Zearalenone, Ochratoxin, DON and Fumonisins) has been developed (Van der Gaag et al., 2003). The detection limits of the multiple assays are 0.2 ng/g for Aflatoxin B1, 0.01 ng/g for Zearalenone, 0.1 ng/g for Ochratoxin A, 50 ng/g for Fumonisin B1 and 0.5 ng/g for DON. For the sample extracts where OTA could be detected, it is possible to increase the sensitivity of the assay using alternative techniques including the detection threshold varing within a range of values ?? below the limit of HPLC detection. These emerging technologies such as biosensors are based on principles of receiving wavelength signals emitted by the molecule on optical surfaces like (e.g. surface plasmon resonance, SPR, and evanescent wave fibre optic) et acoustic (e.g. quartz crystal microbalance).This type of approach has been reported for the determination of Ochratoxin A in liquid food products (Hauck et al., 1998). The assay was based on competition of free Ochratoxin A (test sample) and a sensor surface immobilized conjugate of ochratoxin with the corresponding antibody, the resulting binding of excess antibody to the surface being detected at a resonance frequency of 20 MHz. The method has a linear range of 2 to 100 ng/ml (Patel, 2004). The analytical method based on capillary electrophoresis with fluorescence detection capillary zone electrophoresis coupled with laser-induced fluorescence CZE-LIF) can detect traces of mycotoxins whose resolution was induced by the fluorescent lazer or by exploiting the fluorescent properties of the test molecule and its derivatization with fluoroflore isothiocynate Fluorescein isothiocynate (FITC). This analytical method applied to fumonisins separation and quantification in corn samples involved the following step: after extraction of fumonisins from corn samples with methanol/water, they were isolated using an Immuno Affinity Column. Then the extract was traited by derivatization with FITC and analyzed further by CZE-LIF. The CZE-LIF method was comparable in sensitivity to that of an HPLC method (Maragos et al., 1996). Most methods utilize the fluorescent properties of OTA for subsequent detection and quantification. However, the complex nature of the matrices from which OTA is extracted gives rise to the potential for interference with the fluorescence signal and, therefore many laboratories have used a secondary technique such as liquid chromatography with mass spectrometric detection (LC-MS) to confirm the analytical results obtained from their primary method. Some methods have used mass spectrometry (MS) or immunoassay instead of fluorescence to quantify OTA. For the most part, the recoveries reported using the various methods are above 80 or 90% and detection limits are in the low parts per billion (Scott, 2002).
Fungal diversity observed in 2008 compared to 2009 means that fungi isolated on coffee samples in Ivory Coast would be subject to seasonal and may vary from year to year. But it consists essentially of mycoflora with a predominance of Aspergillus section Nigri. Fungi rate in coffee samples varies with samples types, geographical environment and drying support used. Fungal contamination was probably due to the reduction of water activity which inhibits the growth of other microbial groups e.g. bacteria and yeasts. Humidity and chemical composition of coffee beans, crop’s environmental conditions and product management can influence development of microorganisms and their metabolic activity. The adoption of the Danger and Critical Control Points Analysis System and Good Agricultural Practices (GAP) will significantly influence not only the reduction of fungal contamination risk under the conditions of coffee fruit and bean deterioration but also the reduction of OTA (Batista et al., 2009). The difference in genus diversity can be explained by the difference of homogeneity in the samples. During the 2008 campaign sampling certainly is distinguished by the diversity of the types of samples (dried cherries, green beans and wet and dry shells), sampling sites (plantations, box of farmers, shellers, warehouse) but especially in the area of origin (West and East) coffee samples. This distinction is the more remarkable because the conditions of post-harvest treatment specifically drying and storage are unknown. Sampling during 2009 is more homogeneous than last year because all packages of cherries sampled from a single village in the West, had been taken during drying, whith farmers in the same neighborhood. The change in physico-chemical parameters (temperature, Aw, pH) subjected to weather randomness (alternating rain and sunshine) and the succession of the seasons occurring during post-harvest processing and endogenous flora in the area coffee growing, are sufficient factors to guide the growth of some fungal flora on coffee cherries. Proliferation of fungi in coffee is due to severe faults in harvesting and storage practices like variation of physico-chemical factors (temperature, water activity, humidity) during storage and transport of cherries or coffee beans. It concerns good agricultural practices particularly cherries drying-tickness on drying support (Kouadio et al., 2012), drying process (Velmourougane et al., 2011; Magan and Olsen, 2004) and storage conditions (Bucheli and Tanikawa, 2002). For example in Ivory Coast the effect of coffee cherries quantity put out for sun drying on the kinetics of the drying, fungal growth and Ochratoxin A production was evaluated. The results showed that the more coffee cherries quantity on the drying area was important, the slower they dried. Then the slowness of the drying led to the increasing of fungal development and Ochratoxin A production in the cherries (Kouadio et al., 2012). Their microscopic and macroscopic characteristics are consistent with the description given by (Rapper and Fennell, 1965; Rinyu et al., 1995; Hong et al., 2005; Samson et al., 2006). Aspergillus section Nigri are very widespread in the world. Although their primary source is the ground, they are among the major contaminants of foods and their raw material (wheat, coffee, cocoa) (Perrone et al., 2007). Several species such as A. carbonarius, A. japonicus, A. aculeatus and some variants of the Niger aggregate group are responsible for the post-harvest fruit (apples, peaches, grapes, figs, tomatoes, melons, ...), plant (onions, garlic, yam), and nuts (peanuts, pecans, pistachios, ...) alteration (JECFA, 2002).
Fungal contamination of coffee should not be closely related to mycotoxin production on coffee. To ensure certainty it would be necessary for the determination of OTA in coffee samples. Regarding coffee samples processed by dry method, the results in Table 2 showed that OTA fungi producers have been isolated only from dried coffee cherries. Then if deshusking and despulping are carried out, OTA and fungi contamination in coffee samples would be reduced. Indeed, Batista et al. (2009) concluded as a result of their work that fungi and OTA contamination are concentrated in the skin until processing. According to Bucheli and Tanikawa, (2000), coffee bean skin is the main substrate for the development of OTA fungi. This would explain the fact that potentially ochratoxinogen filamentous fungi were isolated only on dried cherries. Although Ochratoxin A is a mycotoxin of storage, the majority of strains tested in this work, are isolated from coffee cherries during drying in the farms. During storage, in high humidity condition accumulation of toxin is the possible, but the minimum moisture does not guarantee the absence of OTA production. However it takes less moisture for the fungus growth. Many research studies have shown that species of Aspergillus are natural contaminants of coffee and mycotoxins are present on coffee from farming to storage (Nakajima et al., 1997; Silva et al., 2000; FAO, 2006b). Indeed, fungi such as A. ochraceus and Aspergillus section Nigri spp. are responsible for the production of OTA in coffee during drying and storage (Belli et al., 2005). The fungal contamination of the coffee was analyzed by Paterson et al. (2004) in the context of variable climate and evolving. The main climatic factors affecting agriculture in this case the weather variability, seasonality, average rainfall, water availability, and the dynamics and distribution of pests in cases where they are not controlled, as well influence the post-harvest fungal contamination (Bourgeois, 2009). The amount of OTA was dependent on the latitude of the production region: the lower the latitude, the more frequent the occurrence and the greater the concentration. The considerable climatic differences, related to geographic regions, influenced fungi contamination and OTA production in a conclusive manner (Zimmer and Dick, 1996).
Like other foods (cereals, nuts, peanuts, cocoa, dried fruits, cheese, salted and cooked meal, pastry, spicies, ...) (Pfohl-Leszkowicz and Castegnaro 1999) coffee cherries and beans and coffee husk were exposed to fungal contamination during different phases of development, harvesting, preparation, transport and storage. Species were isolated from pure cultures. Phenotypic analysis of the investigated strains in comparison to reference strains do not provide sufficient information to distinguish between species, because of the great phenotypic variability filamentous fungi and especially those of the genera Aspergillus (Raper and Fennell, 1965; Al-Musallam 1980). Indeed molecular methods with DNA-based tools will are useful to examine phylogenetics and systematics of fungi (Bruns et al., 1991; Bowman et al., 1992; Glass and Donalson, 1995). Post-harvest treatment well performed reduces coffee beans fungal contamination. Therefore if the storage takes place in good conditions (good ventilation, optimum moisture, and adequate temperature) green coffee beans were relatively healthy for export. So there were corrections to be made during post-harvest processing of coffee, including the application of good agricultural practice to reduce fungal contamination and mycotoxins production. Also improving the drying process by natural means, including an intermediate stage of pulping and degumming wet may prevent the growth of mold and therefore OTA production during the post-harvest treatment. This will prevent fungal contamination in coffee cherries skin. Because less coffee cherries are infected, the less will be the green coffee beans ready for export and healthy for consumers. Added to this is the search for bioprocess antifungal to fight against the growth of mold on the coffee samples during the post-harvest treatment. In other fungi studied in this chapter have been the subjects of particular study in search of antifungal lactic acid bacteria. The potential antifungal activity of Lactobacillus plantarum against germination and mycelial growth of certain species of Aspergillus section Nigri was confirmed. The results of this work are published in the journal Anaerobe (Djossou et al., 2011). The ageing of coffee plantations has resulted in and the dropping of coffee plantations by the farmers class in favor of new crops export such as cashew tree, Hevea brasiliensis, cotton, oil palm ... . It is of great interest for economic actors in the coffee sector to propose new plant varieties more productive and profitable coffee trees.
The authors have not declared any conflict of interest.
The authors are grateful to French Embassy in Ivory Coast for PhD fellowship. They would like to thanks IRD in France for financial support and the coffee farmers in Ivory Coast for allowing collection in their properties. Our thankfulness is for the National Museum for Natural History in France and the Agricultural Research Service, Southern Regional Research Center in New Orleans, Louisiana for the strains of references classified in international collections.
REFERENCES
|
Albarca ML, Bragulat MR, Castella G, Cabanes FJ (2004). Ochratoxin A Production by Strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 60:2650-2652. |
|
|
|
AOAC (Official Methods of Analysis of the Association of Official Analytical Chemists) (2000). Ochratoxin A in Corn and Barely: Liquid chromatography Method, 17th ed., Arlington, VA; Vol II, Sec. 991.44 |
|
|
|
Al-Musallam A (1980). Revision of the black Aspergillus species. Ph.D. Thesis, State University Utrecht, The Netherlands |
|
|
Batista LR, Chalfoun SM, Ferreira Silva C, Cirillo M, Varga EA, Schwan RF (2009). Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control. 20:784-790.
Crossref |
|
|
Batista LR, Chalfoun SM, Prado G, Schwan RF, and Wheals AE (2003). Toxigenic fungi associated with processed (green) coffee beans (Coffea arabica L.). Int. J. Food Microbiol. 85:293-300.
Crossref |
|
|
Bayman P, Baker JL, Doster MA, Michailides TJ, Mahoney NE (2002). Ochratoxin Production by the Aspergillus ochraceus Group and Aspergillus alliaceus. Appl. Environ. Microbiol. 6(5):2326-2329.
Crossref |
|
|
Belli N, Ramos AJ, Coronas I, Sanchos V, Marin S (2005). Aspergillus carbonarius growth and Ochratoxin A production on a synthetic grape medium in relation to environmental factors. J. Appl. Microbiol. 98:839-844.
Crossref |
|
|
Bennet JW, Klich M (2003). Mycotoxins. Clin. Microbiol. Rev. 16:497–516.
Crossref |
|
|
|
Botton B, Breton A, Fèvre M, Gauthier S, Guy P, Larpent JP, Reymond P, Sanglier JJ, Vayssier Y, Veau P (1990). Moisissures utiles et nuisibles, Importance industrielle, Ed. Masson, Paris, P. 512. |
|
|
|
Bourgeois G (2009). Les dynamiques des cultures et leurs bioagresseurs dans un contexte de climat variable et en évolution. Colloque en phytoprotection. Résistance et approche systémique : nouveaux défis. Centre de référence en agriculture et agroalimentaire du Québec. P. 5. |
|
|
|
Bowman BH, Taylor JW, Brownlee AG, Lee J, Lu S, White TJ (1992). Molecular evolution of the fungi: relationship of the basidiomycetes, ascomycetes and chytridiomycetes. Mol. Biol. Evol. 9:285-296. |
|
|
Bruns TD, White TJ, Taylor JW (1991). Fungal molecular systematics. Ann. Rev. Ecol. Evol. Syst. 22:525-564.
Crossref |
|
|
Bucheli P, Tanikawa MH (2002). Research on the origin, and on the impact of post-harvest handling and manufacturing on the presence of ochratoxin A in coffee. Food Addit. Contam. 19:655–665.
Crossref |
|
|
Bragulat MR, Abarca ML, Cabanes FJ (2001). An easy screening method for fungi producing Ochratoxin A in pure culture. Int. J. Food Microbiol. 7:139-144.
Crossref |
|
|
|
Cahagnier B, Richard-Molard D (1998). Analyse mycologique in Moisissures des aliments peu hydratés, Ed. Tec & Doc, Paris pp. 140-158. |
|
|
Castegnaro M, Canadas D, Vrabcheva T, Petkova-Bocharova T, Chernozemsky IN, Pfohl-Leszkowicz A (2006). Balkan endemic nephropathy: role of Ochratoxin A through biomarkers. Mol. Nutr. Food Res. 50 (6):519-529.
Crossref |
|
|
|
Commission du Codex Alimentarus (CCA) (2007). Programme mixte fao/who sur les normes alimentaires. Comité du codex sur les contaminants dans les aliments. Cx/cf 07/1/18. |
|
|
|
Clenny Mc N (2005). Laboratory detection and identification of Aspergillus species by microscopic observation and culture: the traditional approach. Med. Mycol. 1(43):S125/S128. |
|
|
Djossou O, Perraud-Gaime I, Lakhal-Mirleau F, Rodriguez-Serrano G, Karou G, Niamke S, Ouzari I, Boudabous A, Roussos S (2011). Robusta coffee beans post harvest microflora: Lactobacillus plantarum sp. as potential antagonist of Aspergillus carbonarius. Anaerobe 17:267-272.
Crossref |
|
|
|
Dano Djédjé S, Manda P, Kouadio JH, Diakité A, Droh KJ, Kouassi KS, Dembélé A (2009). Etude de L'incidence de la Torréfaction Appliquée au Café Vert Sur la Réduction du Taux de L'Ochratoxine A (OTA) dans le Produit Fini. E. J. Sci. Res. 26(3):393-401. |
|
|
|
De Hoog GS, Guarro J (1995). Atlas of clinical fungi, Centraalbureau voor Schimmelcultures, Baarn, Pays-Bas. |
|
|
|
EFSA (European Food Safety Authority) (2006). Opinion of the scientific panel on contaminants in food chain on a request from the commission related to Ochratoxin A in food. EFSA J. 365:1-56. |
|
|
|
Europeenne Union (EU) (2010). Règlement UE N°105/2010 de la Commission du 5 février 2010. J.O.UE L 35/7. |
|
|
|
FAO (2006a). Enhancement of coffee quality through the prevention of mould formation. Final Technical Report. Final Management Report, Julius Jackson (Project Officer, FAO). |
|
|
|
FAO (2006b). Food and Agriculture Organization of United Nations. Reducing Ochratoxin A in Coffee. |
|
|
|
Filtenborg O, Frisvad JC (1980). A simple screening method for toxigenic moulds in pure cultures. Lebensmittel-Wissenschaft und Technologie 13:128–130. |
|
|
|
Glass NL, Donaldson GC (1995). Development of Primer Sets Designed for Use with the PCR To Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 61(4):1323-1330. |
|
|
|
Hauck S, Kosslinger C, Drost S and Wolf H (1998), Biosensor system to determine Ochratoxin A. Lebensmittelchemie 52:58. |
|
|
Hong SB, Go SJ, Shin HD, Frisvad JC, Samson RA (2005). Polyhphasic taxonomy of Aspergillus fumigatus and related species. Mycologia. 97:1316-1329.
Crossref |
|
|
|
JECFA (Joint Expert Committee on Food Additives) (2002). Evaluation of Certain Mycotoxins in Food: Fifty-sixth Report of the Joint FAO/WHO Expert Committee on Food Additives. (WHO Technical Report Series No 906) ISBN 9241209062 |
|
|
Joosten HMLJ, Goetz J, Pittet A, Schellenberg M, Bucheli P (2001). Production of Ochratoxin A by Aspergillus carbonarius on coffee cherries. Int. J. Food Microbiol. 65:39-44.
Crossref |
|
|
Kouadio IA, Koffi LB, Nemlin JG, Doss MB (2012). Effect of Robusta (Coffea canephora P.) coffee cherries quantity put out for sun drying on contamination by fungi and Ochratoxin A (OTA) under tropical humid zone (Côte d'Ivoire). Food Chem. Toxicol. 50(6):1969-1979.
Crossref |
|
|
|
Kouadio AI, Agbo NG, Dosso BM (2010). Diagnostic de la contamination des cerises de café Robusta (Coffea canephora P.) par les espèces fongiques productrices de l'Ochratoxin A en zone tropicale humide (Côte D'Ivoire). Annuaire Botanique de l'Afrique Ouest. 6:14-26. |
|
|
|
Kuiper-Goodman T (1998). Food safety: mycotoxins and phycotoxins in perspective. In: Miraglia M, Van Edmond H, Brera C, Gilbert J (Eds.), Mycotoxins and Phycotoxins-Developments in Chemistry, Toxicology and Food Safety. Proceedings of the IX IUPAC International Symposium. Alaken Inc., Collins, CO, pp. 25-48. |
|
|
Krska R (1998). Performance of modern sample preparation techniques in the analysis of Fusarium mycotoxins in cereals. J. Chromatogr. A. 815:49-57.
Crossref |
|
|
Leong SL, Hien LT, An TV, Trang NT, Hocking AD, Scott ES (2007). Ochratoxin-A producing Aspergillus in Vietnamese green coffee beans. Lett. Appl. Microbiol. 45:301-306.
Crossref |
|
|
Magnani M, Fernandes T, Prete CSEC, Homechim M, Ono EYS,Vilas-Boas LA, Sartori D, Furlaneto, MC, Fungaro MHP (2005). Molecular identification of Aspergillus spp. isolated from coffee beans. Sci. Agric. 62:45-49.
Crossref |
|
|
Magnoli C, Astoreca A, Ponsone ML, María GF, Carla B, Ana MD (2007). Ochratoxin A and Aspergillus section Nigri in peanut seeds at different months of storage in Córdoba, Argentina. Int. J. Food Microbiol. 119:213-218.
Crossref |
|
|
Maragos CM, Bennett GA, Richard JL (1996), Analysis of fumonisin B1 in corn by capillary electrophoresis, in Jackson L S, DeVries J W and Bullerman L B, Fumonisins in Food, New York, Plenum Press pp. 105–112.
Crossref |
|
|
|
Marquardt RR, Frohlich AA (1992). A review of recent advances in understanding ochratoxicosis. J. Anim. Sci. 70(12):3968-88. |
|
|
Martins ML, Martins HM, Gimeno A (2003). Incidence of microflora and of Ochratoxin A in green coffee beans (Coffea arabica). Food Addit. Contam. 20:1127-1131.
Crossref |
|
|
Nakajima M, Tsubouchi H, Miyabe M, Ueno Y (1997). Survey of Aflatoxin B1 and Ochratoxin A in commercial green coffee beans by high-performance liquid chromatography linked with immunoaffinity chromatography. Food Agric. Immunol. 9(2):77-83.
Crossref |
|
|
Noonim P, Mahakarnchanalkul W, Nielsen KF, Frisvad JC, Samson RA (2008). Isolation, identification and toxigenic potential of Ochratoxin A-producing Aspergillus species from coffee beans grown in two regions of Thaïland. Int. J Food Microbiol. 128(2):197-202.
Crossref |
|
|
Pandey A, Soccol CR, Poonam N, Brand D, Radjiskumar M, Roussos S (2000). Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 6(2):153-162.
Crossref |
|
|
|
Pardo E, Marin S, Ramos A, Sanchis V (2004).Occurrence of ochratoxigenic fungi and Ochratoxin A in green coffee from different origins. Food Sci. Technol. Int. 10(1):45-49.
Crossref
|
|
|
|
Patel P (2004) Mycotoxins analysis: current and emergency technologies Chap.5 p.88, Part I Measuring risks, Leatherhead Food International Ltd, UK. |
|
|
Perrone G, Susca A, Cozz G, Ehrlich K, Varga J, Frisvad JC, Meijer M, Noonim P, Mahakarnchanakul W, Samson RA (2007). Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 59:53-66.
Crossref |
|
|
|
Pfohl-Leszkowicz A, Castegnaro M (1999). Ochratoxine. In: Lavoisier, Tec & doc, Mycotoxines: Evaluation et gestion du risque. pp. 249-267. Lavoisier, Paris, France. |
|
|
|
Pitt JI (2000). Toxigenic fungi: which are important? Med. Mycol. 38(1):17-22. |
|
|
Pittet A, Royer D (2002). Rapid, Low Cost Thin-Layer Chromatographic Screening Method for the Detection of Ochratoxin A in Green Coffee at a Control Level of 10µg/kg J. Agric. Food Chem. 50:243−247.
Crossref |
|
|
|
Raper KB, Fennell DI (1965). The genus Aspergillus. Williams and Wilkins Company, Baltimore. |
|
|
|
Raquel Duarte da CCB, Thais Matsue U, Passos da Cunha C, Andrade Costa D, Henrique Campino de la Cruz M, Ronoel Luiz de Oliveira G, Valnei SCJRM (2008). Development of Method for Ochratoxin A Analysis in Coffee by Liquid Chromatography/Electrospray Tandem Mass Spectrometry. Simposio de Metrología Santiago de Querétaro, México, 22 al 24 de Octubre. Centro Nacional de Metrología SM2008-M103-1033-1. |
|
|
Riba A, Mokrane S, Mathieu F, Lebrihi A, Sabaou N (2008). Mycoflora and Ochratoxin A producing strains of Aspergillus in Algerian wheat. Int. J. Food Microbiol. 122:85-92.
Crossref |
|
|
|
Rinyu E, Varga J, Ferenczy L (1995). Phenotypic and genotypic analysis of variability in Aspergillus fumigatus. J. Clin. Microbiol. 33(10):2567-2575. |
|
|
Roussos S, Nabila Z, Ghislane S A Tantaoui-E, Khadija L, Mostafa C, Hicham H, Frederic V, Perraud-Gaime I, Augur C, Ismaili-Alaoui M (2006). Characterization of filamentous fungi isolated from Moroccan olive and olive cake: Toxinogenic potential of Aspergillus strains. Mol. Nutr. Food Res. 50(6):500-506.
Crossref |
|
|
Salma L, Neus B, Samir C, Zghonda N, Mliki A, Vicente S, Abdelwahed G (2007). Occurrence of ochratoxigenic fungi and Ochratoxin A in grapes from a Tunisian vineyard. Int. J. Food Microbiol. 114:376-379.
Crossref |
|
|
|
Samson RA, Houbraken JAMP, Kuijpers AFA, Frank JM, Frisvad JC (2004). New ochratoxin or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 50:45–61. |
|
|
Samson RA, Hong SB, Frisvad JC (2006). Old and new concepts of species differentiation in Aspergillus. Med. Mycol. 44:S133-S148.
Crossref |
|
|
Samson RA, Noonim P, Meijer M, Houbraken J, Frisvad JC, Varga J (2007). Diagnostic tools to identify black Aspergillus. Stud. Mycol. 59:129-145.
Crossref |
|
|
Sanchez-Hervas M, Gil JV, Bisal F, Ramon D, Martinez-Culebras PV (2008). Mycobiota and mycotoxin producing fungi from cocoa beans. Int. J. Food Microbiol. 125:336-340.
Crossref |
|
|
Schwan RF, Wheals AE (2003). Mixed microbial fermentations of chocolate and coffee. In: Vincent Robert. (Org.) Yeasts in Food. Hamburg, Alemanha: Behr´s Verlag 1:426-459. Science. 70(12):3968-3988.
Crossref |
|
|
Scott PM (2002). Methods of analysis for Ochratoxin A, Adv. Exp. Med. Biol. 504:117–34.
Crossref |
|
|
|
Magan N and Olsen M (2004). Mycotoxins in Food-Detection and control. part III Particular mycotoxins, Chap.13 Ochratoxin A. P.307, (Eds). Woodhead Publishing ISBN 1 85573 733 7 (book) 1 85573 908 9 (e-book) in Food science and technology. |
|
|
Silva CF, Schwan RF, Dias ES, Wheals AE (2000). Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 60:251-260.
Crossref |
|
|
Schmidt-Heydt M, Bode H, Raupp F, Geisen R (2010). Influence of light on ochratoxin biosynthesis by Penicillium. Mycotox. Res. 26:1-8.
Crossref
PMid:23605235 |
|
|
Taniwaki MH, Pitt JI, Texeira AA, Iamanaka BT (2003). The source of Ochratoxin A in Brazilian coffee and its formation in relation to processing methods. Int. J. Food Microbiol. 82:173-179.
Crossref |
|
|
Teren J, Varga J, Hamari Z, Rinyu E, Kevel F (1996). Immunochemical detection of Ochratoxin A in black Aspergillus strains. Mycopathologia 134:171-176.
Crossref |
|
|
|
Tournas V, Stack ME, Mislivec PV, Koch HA, Bandler R (2001). Bacteriological Analytical Manual Online. US Food & Drug Administration. |
|
|
Van der Gaag B, Spath S, Dietrich H, Stigter E, Boonzaaijer G, Van Osenbruggen T, Koopal K (2003). Biosensors and multiple mycotoxin analysis. Food Control 14:251–254.
Crossref |
|
|
Van der Merve KJ, Steyn PS, Fourie L (1965). Mycotoxins. Part II. The constitution of Ochratoxins A, B and C; metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. 36:7083-7088.
Crossref |
|
|
|
WHO (2002). List of priority Contaminants and Commodity Combinations.
View
|
|
|
Zimmerli B, Dick R (1996). Ochratoxin A in table wine and grape-juice: occurrence and risk assessment. Food Additives Contaminants, 13:655–668.
Crossref |