ABSTRACT
Herbicides can cause negative effects on the morphophysiological characteristics of agricultural crops when proper knowledge about their selectivity is not available. For erva-mate plantations, herbicides which are registered and recommended are scarce. In this sense, this study was carried out to evaluate the selectivity of herbicides applied in different doses to erva-mate. The experiment was installed in a greenhouse at the Federal University of Fronteira Sul (UFFS), Campus Erechim, state of Rio Grande do Sul (RS), Brazil. The experimental design was a randomized block, arranged in a 7 × 4 factorial scheme, with four replications. Factor A comprised the herbicides (tembotrione, chlorimuron-ethyl, oxyfluorfen, sethoxydim + diclosulam, metsulfuron-methyl, Fomesafen + fluazifop-p-butyl and nicosulfuron) and factor B comprised the doses of these herbicides (0, 0.5, 1 and 2‑fold the recommended dose for other crops, on the respective herbicide labels). The phytotoxicity, plant height, stem diameter, dry mass of shoots and roots, chlorophyll content, sub-stomatal CO2 concentration, photosynthetic rate, CO2 consumed, stomatal conductance of water vapors, transpiration rate, and water use efficiency were assessed. Oxyfluorfen, fomesafen + fluazifop-p-butyl and nicosulfuron affected the morphophysiological characteristics of erva-mate plants at all doses tested. The chlorimuron-ethyl and sethoxydim + diclosulam were presented as being potential to be used in erva-mate plantations until 2‑fold the label dose, because they present low plant toxicity and reduced interference in the morphophysiological characteristics of erva-mate.
Key words: Chlorimuron-ethyl, alternative crop, chemical management.
The erva-mate (Ilex paraguariensis St. Hil.) is a native tree species in South America. It has80%ofitsnatural occurrence in Brazil (Cardozo Jr. et al., 2010) where it is called “erva‑mate”, playing an important economic and social role, especially for smallholders. Despite the importance of this plant for the economy, their productivity of 7,650 kg ha‑1 or 510 ha‑1 (Seab, 2014) is far below what could actually be produced, due to the presence of factors limiting the growth and development of the erva-mate plants. According to Agostinetto et al. (2010), forest crops like any plant community are subjected to a series of ecological factors that directly or indirectly can affect the growth of trees, and in the case of erva-mate, the production of leaves. Among these factors, weed interference which leads to productivity losses due to the competition for environmental resources and allelopathy, may be highlighted.
These factors also interfere with the quality of the harvested product (Vargas and Roman, 2005). Toledo et al. (2000) reported that weed management in reforestation is carried out by mechanical and chemical controls, isolated or combined. The mechanical control is basically done by weeding and brushing, with the advantage of causing little or no injury to the plantation. On the other hand, the widespread use of the chemical weed control is due to the cultivation of large areas, practicity, efficiency, low cost, and mainly the lower demand for human labor compared to other weed control methods. However, just a few herbicides are registered for the selective post‑emergence weed control in erva-mate (MAPA, 2017), and studies regarding the selectivity of herbicides to this crop are scarce.
The negative effects of herbicides when applied on cultivated plants are important aspects that must be considered, since they can influence several physiological processes, consequently reflecting on the quantity and the quality of the harvested product. Intoxication effects of herbicides to plants should not be determined solely by verifying the visual symptoms, as examples of herbicides that may reduce crop productivity without causing visually detectable effects are known. On the other hand, some herbicides can cause severe injuries, which disappear with the development of the crop with little or no impact on productivity (Velini et al., 2000; Negrisoli et al., 2004).Several factors may influence the growth and development of the crop, which is largely determined by the photosynthetic rate of the plant.
This parameter is directly or indirectly influenced by water deficiency, thermal stress (Loreto and Bongi, 1989), internal and external gas concentration on the leaf environment (Kirschbaum and Pearcey, 1988), composition and light intensity (Sharkey and Raschke, 1981), and mainly stresses caused by the application of herbicides. However, studies related to the effect of herbicides on the physiology of erva-mate are scarce and the effect of these products on the crop has to be deeply reported. Thus, in order to adopt the chemical weed control method in erva-mate plantations, it is necessary to evaluate the selectivity of commonly available herbicides to these plants. The hypothesis is that the selectivity of herbicides to erva-mate plants is a function of product and dose. Thus, this study was carried out to assess the selectivity of herbicides applied at different doses to erva-mate plants.
Study species
The erva‑mate (I. paraguariensis) is a tree native to the subtropical regions of South America. Its leaves and thin branches are toasted and consumed as hot or cold tea, which are called “chimarrao” and “terere”, respectively. This tree usually reaches 12 m in height, being relatively sensitive to excessive sunlight, demanding some degree of shading in its initial establishment.
Greenhouse trial
The experiment was carried out in a greenhouse at the Federal University of Fronteira Sul (UFFS), at the campus located in the city of Erechim, state of Rio Grande do Sul (RS), Brazil, in a randomized complete block design arranged in a 7 × 4 factorial scheme, with four replications. Factor A comprised the herbicides (tembotrione, chlorimuron-ethyl, oxyfluorfen, sethoxydim + diclosulam, metsulfuron-methyl, fomesafen + fluazifop-p-butyl and nicosulfuron) (MAPA, 2017), and factor B comprised the doses of these herbicides (0, 0.5, 1 and 2‑fold the recommended dose for other crops, on the respective herbicide labels) (Table 1).
Young plants of erva-mate with uniform size of20 ± 2 cm, native genotype from the location of Erechim, RS, Brazil, were selected from the same planting lot. The experimental units were composed of polyethylene pots with capacity of 8 dm3, filled with red latosol aluminoferric, where one plant was transplanted to the center in the first half of May, 2015. Fertilization was used in accordance with the technical recommendations for cultivation of erva-mate (Rolas, 2004) 15 days after transplanting (DATp), based on soil analysis. Plants were protected with a 50% shading screen in the first 30 DATp. The application of the herbicides was performed directly to the plants 60 DATp by using a CO2 pressurized backpack sprayer connected to a single spray tip of the series TT 110.02, operating at 2.0 kgf cm‑2 with volume equivalent to 150 L ha‑1.
Phytotoxicity was evaluated at 7, 14, 21, 28, 35, 42 and 49 days after application of the treatments (DAT) being assessed visually, assigning scores from zero to 100% by two evaluators, where zero (0%) corresponds to no injury and 100 (100%) to death of plants, according to the methodology proposed by SBCPD (1995). Fifty DAT plant height (cm) was assessed with a ruler, from soil to the apical meristem; stem diameter (cm) was determined with a digital caliper rule of 5 cm above soil and chlorophyll content (SPAD) was assessed with a digital SPAD meter, evaluating leaves in the lower, middle and upper third of plants.
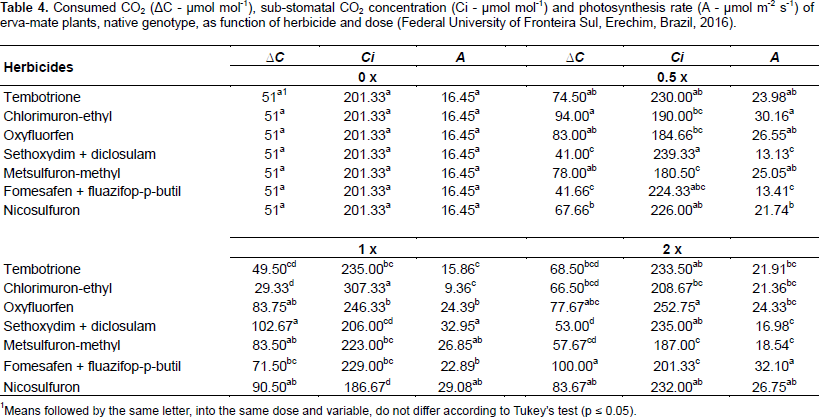
Physiological parameters were assessed at 51 DAT: sub‑stomatic CO2 concentration (Ci - μmol mol‑1), photosynthetic rate (A - μmol m‑2 s‑1), CO2 consumed (ΔC - µmol mol‑1), water use efficiency (WUE - mol CO2 mol H2O‑1), and transpiration rate (E - mol H2O m‑2 s‑1). These variables were determined in the middle third of the first fully expanded leaf of the erva-mate plants, by using an infrared gas analyzer (IRGA), ADC-LCA PRO (Analytical Development Co. Ltd, Hoddesdon, UK); each block was assessed per day, between 8 and 10 o'clock in the morning to guarantee homogeneous environmental conditions during the analysis of each block. Seventy DAT plants were removed from the vases, sectioned with roots and shoots separated, packed in paper bags and put into forced air circulation oven at 65 ± 5°C for four days, to determine the dry mass.
Statistical analysis
The data set was submitted for analysis of variance by the F‑test and for the quantitative factor (doses), linear and non-linear regressions were adjusted. Tukey’s test was used for the qualitative factor (herbicides). All tests were performed at 5% probability.
There was interaction between herbicide and dose for all variables. There was increase in phytotoxicity as herbicide rates were increased, mainly at 7 DAT for doses of fomesafen + fluazifop-p-butyl, with the highest injury occurring with twice the recommended dose (250 + 250 g ha‑1). The application of sethoxydim + diclosulam had no considerable phytotoxicity for any dose, stabilizing in about 4% (Figure 1A). For the other herbicides, there were no model adjustments to the data, but the mean phytotoxicity exceeded 6.42%. It should be noted however that, the low levels of phytotoxicity found at 7 DAT is due to the fact that, depending on the mechanism of action to which the herbicide belongs, there is a need for more time forit to show injury to the plants in which they are applied (Rodrigues and Almeida, 2011).
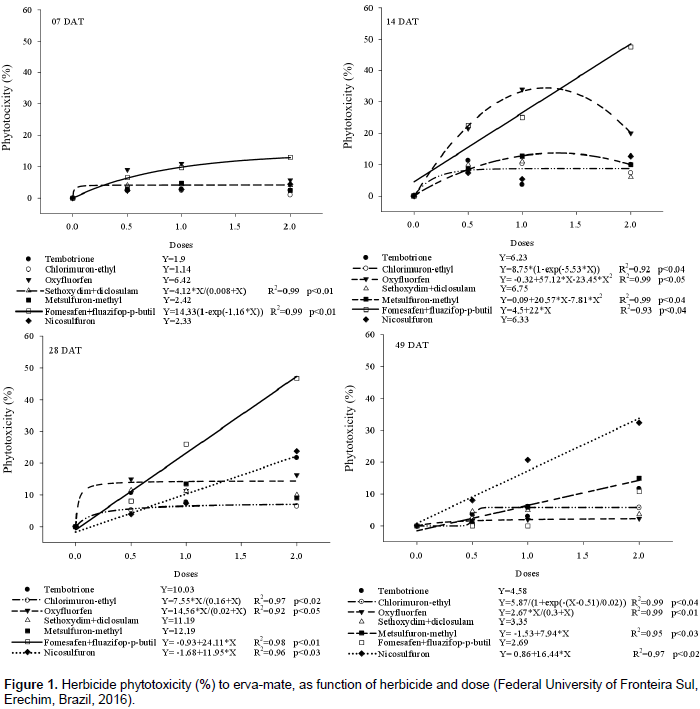
It should be noted that fomesafen belongs to the mechanism of PROTOX inhibition, with action on broad and narrow leaves, whereas fluazifop-p-butyl inhibits the enzyme acetyl-CoA carboxylase (ACCase) and therefore is a specific grass killer. Thus, the phytotoxic effects of the fomesafen + fluazifop-p-butyl mixture can be attributed mainly to fomesafen. At 14 DAT, all herbicides showed increased levels of plant damage (Figure 1B) compared to the first evaluation. At 28 DAT (Figure 1C), fomesafen + fluazifop-p-butyl maintained high phytotoxicity levels. In the last phytotoxicity assessment carried out 49 DAT, the herbicide nicosulfuron presented the highest rate of injury to erva-mate, reaching about 17 and 34% when using the dose and 2‑fold the recommended dose, respectively. Similarly to nicosulfuron, metsulfuron-methyl showed a linear increase of about 16% in injury to plants as dose increased, with application of 2‑fold the recommended dose (Figure 1D).
It was observed that oxyfluorfen and chlorimuron-ethyl caused phytotoxicity between 1 and 5%, even at high doses. There was no regression fit for the increasing doses of tembotrione, sethoxydim + diclosulam and fomesafen + fluazifop-p-butyl (Figure 1D), with averages of 5, 3 and 3% phytotoxicity, respectively. It was observed that in cases where there was phytotoxicity to the plants, the recovery relied on the emission of new sprouts from the damaged shoots. For the herbicides that present contact effect, new sprouts that appear after the application were not affected (Oliveira Júnior et al., 2011), provided that the injury is not high to the point of causing the death of the growth meristems.
Carotenoid inhibiting herbicides, in general terms, act efficiently in pre‑ or early post‑emergence of plants (Vidal and Merotto, 2001). The fact that the erva-mate is transplanted with a more developed vegetative structure guaranteed plant recovery at 35 DAT with tembotrione.
Although the herbicides chlorimuron-ethyl, metsulfuron-methyl, nicosulfuron and diclosulam are all inhibitors of the enzyme acetolactate synthase (ALS), they showed distinct behavior. Chlorimuron-ethyl, metsulfuron-methyl and sethoxydim + diclosulam showed phytotoxicity below 15% for erva-mate plants (Table 2). This can be explained by the fact that plants were probably able to metabolize the herbicide at such a rate that it prevents it from reaching the site of action (Vidal and Merotto, 2001). Nicosulfuron at 49 DAT showed up to 35% phytotoxicity at 2‑fold the label dose (Table 2).There was fitting for plant height only for doses of chlorimuron-ethyl and metsulfuron-methyl. For both, plant growth stagnation was about 27 and 45% after application of half and the full dose, respectively compared to the control (Figure 2). When comparing herbicides within each dose, nicosulfuron showed lower plant height at all doses, not differing from tembotrione, chlorimuron, sethoxydim + diclosulam and metsulfuron-methyl. The highest effect was verified with the use of 2‑fold the dose, when plant height reduction was approximately 56% compared to control plants (Table 3).
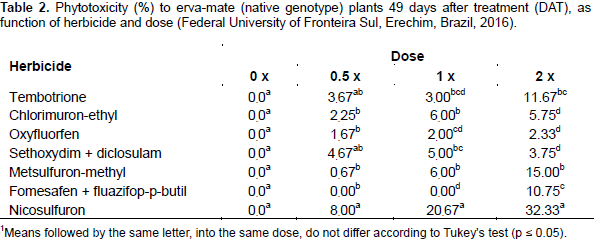

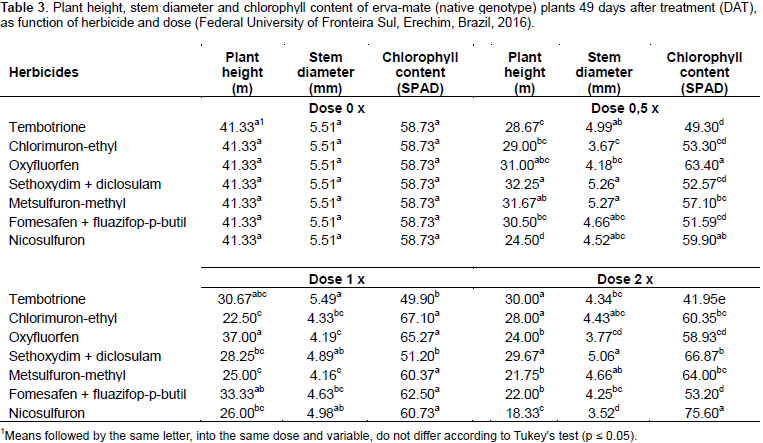
For stem diameter, there was no curve fitting (data not shown). In the comparison between herbicides and doses, nicosulfuron showed the lowest stem diameter (3.52 mm) when under application of 2‑fold the dose (120 g ha‑1), which was about 36% less than the control (Table 3). The stagnation in plant height, as well as the remarkable phytotoxicity, also affected the stem diameter of plants treated with nicosulfuron, but not its chlkorophyll content (Figure 3). With regard to the physiological variables, sub-stomatal CO2 concentration, photosynthetic rate, CO2 consumed, stomatal conductance, transpiration rate and water use efficiency, it was possible to adjust models only for stomatal conductance (Gs), transpiration rate (E), and water use efficiency (Figure 4).

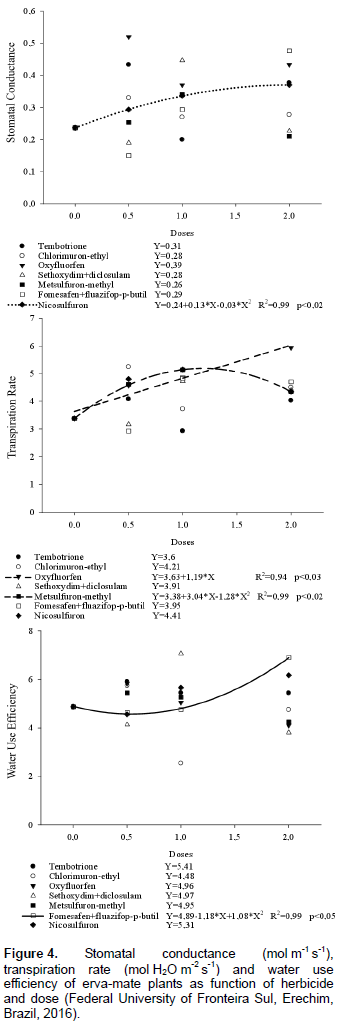
For nicosulfuron, an increase in Gs of 24, 42 and 58% was observed with the use of 0.5, 1.0 and 2.0‑fold the dose, respectively, compared to the control (Figure 4). It can be verified that the Gs increase is proportional to the phytotoxicity increase caused by nicosulfuron at 49 DAT. When comparing the effect of the herbicides within each dose, it was observed that oxyfluorfen and nicosulfuron showed higher rates of Gs compared to the other herbicides at all doses. However, twice the dose of oxyfluorfen and fomesafen + fluazifop-p-butyl increased Gs from 0.43 to 0.48 mol m‑1 s‑1, superior to the other herbicides, but not differing from tembotrione and nicosulfuron (Table 4).
For oxyfluorfen and fomesafen+fluazifop-p-butyl, the high rates of Gs are related to the accelerated metabolism of plants for the emission of new sprouts, since the contact effects of these herbicides do not act on new vegetative formations. The results corroborate with those reported by Concenço et al. (2014), who reported that 25% of the dose of fluazifop-p-butyl, clethodim and the commercial mixture of bentazon + imazamox, promoted increases in stomatal conductance of Crambe abyssinica.
For the transpiration rate (E), linear and quadratic models were adjusted for oxyfluorfen and metsulfuron-methyl, respectively (Figure 4). For oxyfluorfen, the increase in doses resulted in a linear increase in the E of erva-mate; with double the dose there was increase of about 66% in the evaluated variable (Figure 4). Metsulfuron-methyl showed a distinct behavior, with an increase of approximately 52% in E when the recommended dose (3.6 g ha‑1) was applied, compared to control (Figure 4). However, with 2‑fold dose, an increase of about 28% was observed. The differentiated behavior of oxyfluorfen and metsulfuron-methyl with respect to E of erva-mate can be associated with the different mechanisms of action of these herbicides.
As the E is mainly determined by Gs, it can be hypothesized that there was an increase in the metabolism and in E for the emission of new sprouts under application of oxyfluorfen (contact herbicide) and increase in metabolism for detoxification of metsulfuron-methyl. Similar results were found by Concenço et al. (2014), who reported increase in E of C. abyssinica with 75% of the dose of bentazon + imazamox compared to the lowest dose tested. Similarly, Galon et al. (2014) found that ryegrass plants showed an increase in Gs and E rates when they grew under application of the recommended dose of imazethapyr + imazapic.
Regarding water use efficiency, fomesafen + fluazifop-p-butyl presented quadratic behavior as a function of dose increase. It was observed that the WUE practically did not change until the recommended dose, however with the application of 2‑fold the dose, an increase of approximately 40% was verified (Figure 4). Fluazifop-p-butyl is an inhibitor of the enzyme ACCase and has no effect on dicotyledons (Vidal and Merotto, 2001), while fomesafen, as a contact-effect herbicide (Oliveira Júnior et al., 2011) destroys mainly meristematic tissues. The commercial mixture of fomesafen + fluazifop-p-butyl caused high phyto toxicity 07 to 42 DAT; the largest WUE for this treatment occurred as the plants showed intense development of new apical shoots after 42 DAT.
In general, oxyfluorfen and fomesafen + fluazifop-p-butyl reached the highest rates of sub-stomatal CO2 (Ci), photosynthetic rate (A) and CO2 consumed (ΔC) at all doses evaluated (Table 4). However, with application of the label dose, sethoxydim + diclosulam presented higher ΔC and A, not statistically differing from metsulfuron-methyl and nicosulfuron. Chlorimuron-ethyl and nicosulfuron, at the label dose, had respectively the highest and lowest Ci, with the other herbicides being intermediary.
The selectivity of herbicides to plants of the same genotype may be related to several factors like the physiological detoxification capacity of some plants, the environmental influences and also the physical, chemical and biological characteristics of the herbicides. Brandão et al. (2014) reported that the greater the genetic variability within the same species, the greater the diversity of responses that may occur. A reduction was observed in the accumulation of shoot dry mass of erva-mate with the increase of the doses of oxyfluorfen and fomesafen + fluazifop-p-butyl; for the other herbicides, it was not possible to adjust a regression to the data. Oxyfluorfen caused a fall in the dry mass accumulation of about 26,42 and 45% when applied, respectively at 0.5 , 1 and 2 fold the recommended doses (Figure 5A). Fomesafen + fluazifop-p-butyl was shown to be more phytotoxic to the crop, especially when applying 2 fold the dose (250 + 250 g ha 1).

Compared with the control that produced around 16 g of dry mass per plant, the application of 2‑fold the dose of fomesafen + fluazifop-p-butyl accumulated 5.3 g plant‑1, that is 67% drop in the variable (Figure 5A). When comparing herbicides used at twice the dose, it is noted that the lowest accumulation of shoot dry mass occurred when oxyfluorfen was used, but did not differ statistically from fomesafen + fluazifop-p-butyl and nicosulfuron. At the same dose, the herbicide that presented the highest shoot dry mass was chlorimuron-ethyl, not differing from tembotrione (Table 5). The lower accumulation of dry mass is directly related to dose increase of herbicides, making an inversely proportional relation. Oxyfluorfen, fomesafen + fluazifop-p-butyl, metsulfuron-methyl and nicosulfuron caused greater phyto toxicity with necrosis, death of meristems and leaf abscission. In general, the application of herbicides reflected in lower dry mass of shoots in erva-mate plants.
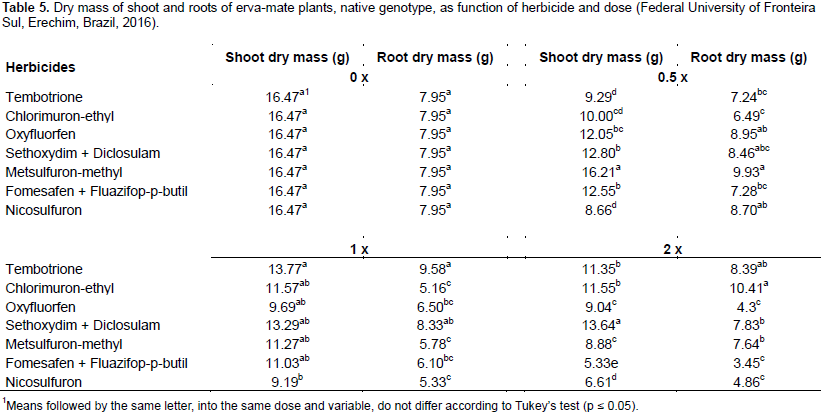
Root dry mass presented similar results to the shoot dry mass (Figure 5B). Reduction of root dry mass was observed with increasing dose of fomesafen + fluazifop-p-butyl; the application of 0.5‑f, 1‑ and 2‑fold the label dose resulted in a reduction of approximately 14, 28 and 56% accumulation of root dry mass, respectively for the doses (Figure 5B). The herbicides that caused the most damage tothe plant root system were fomesafen + fluazifop-p-butyl, nicosulfuron and oxyfluorfen, which did not differ statistically in the highest dose tested (Table 5). It can be inferred that the contact effect of the herbicide reduced the photo synthetically active area and consequently the production of photo assimilates, directly influencing root development.
Brighenti and Muller (2014) observed reductions of up to 86% of Australian cedar root dry mass under the effect of different herbicide doses. Similar results were found by Brandão et al. (2014), who observed reduction of up to 50% in root dry mass of açaí plants that received application of herbicides in 3‑leaf stage. Tuffi‑Santos et al. (2006) found a decrease of approximately 60% in the root dry mass of Eucalyptus under application of 172 g ha‑1 of glyphosate. The herbicides interfere differently with erva-mate development. The increase in doses caused an increase in phyto toxicity, mainly for oxyfluorfen and fomesafen + fluazifop-p-butyl until 28 DAT.
After this period, metsulfuron-methyl and nicosulfuron were more phytotoxic, with long-lasting effects so that at the end of the evaluated period (49 DAT) the lowest phyto toxicity was observed in plants applied with chlorimuron-ethyl and sethoxydim + diclosulam, which even at the highest dose did not cause more than 12% phyto toxicity. On the other hand, the highest level of injury was found mainly with the use of oxyfluorfen, fomesafen + fluazifop-p-butyl and nicosulfuron. In an attempt to detoxify the herbicides and emit new sprouts, an increase in plant metabolism and acon sequent increase in the physiological characteristics were observed. As the erva-mate is a plant that has not yet under gone genetic improvement processes thus presenting high heterogeneity, there is a need to carry out additional studies, especially concerning herbicide selectivity.
The authors have not declared any conflict of interests.
The authors appreciate CNPq and FAPERGS for the scholarships and financial support to this research.
REFERENCES
|
Agostinetto D, Tarouco CP, Markus C, Oliveira E, Silva JMBV, Tironi SP (2010). Seletividade de genótipos de eucalipto a doses de herbicidas. Semina: Ciênc. Agrár. 31:585-598. |
|
|
|
Brandão BB, Costa SJ, Nunes DP, Marinho GA, Erasmo EAL (2014). Selectivity of herbicides on the growth of initial culture of açaí (Euterpe oleracea Mart.). J. Biotechnol. Biodiv. 5:95-100. |
|
|
Brighenti AM, Muller MD (2014). Tolerância de plantas de Khaya ivorensis e Toona ciliata a herbicidas. Floresta 44:747-554.
Crossref |
|
|
Cardozo Junior EL, Donaduzzi CM, Ferrasere Filho O, Friedrich, JC, Gonela A, Sturion JA (2010). Qualitative genetic analysis of methylxanthines and phenolic compounds in mate progênies. Pesqui. Agropecu. Bras. 45:171-177.
Crossref |
|
|
|
Concenço G, Ferreira EA, Marques RF, Nunes TC, Santos SA, Palharini WG, Marschall IR, Alves MES, Mendonça CG (2014). Características fisiológicas de Crambe abyssinica sob aplicação de herbicidas. Rev. Ciênc. Agrár. 37:361-369. |
|
|
|
Galon L, Guimaraes S, Lima AM, Concenço G, Krolow IRC, Ferreira EA (2014). Influência de herbicidas do grupo das imidazolinonas em características fisiológicas de plantas cultivadas no inverno. Pesqui. Agropecu. Gaúcha 20:41-50. |
|
|
Kirschbaum MUF, Pearcey RW (1988). Gas exchange analysis of the relative importance of stomatal and biochemicalfactors in phosynthetic induction in Alocasia macrorrhiza. Plant Physiol. 86:782-785.
Crossref |
|
|
Loreto F, Bongi G (1989). Combined low temperature-high light effects on gas exchange properties of jojoba leaves. Plant Physiol. 91:1580-1585.
Crossref |
|
|
|
Ministério da Agricultura, Pecuária e Abastecimento (MAPA) (2017). Agrofit. Available in: <http://agrofit. Agricultura.gov.br/agrofit_cons/principal_agrofit_cons>. Access on: February 2, 2017. |
|
|
Negrisoli E, Velini ED, Tofoli GR, Cavenaghi AL, Martins D, Morelli JL, Costa AGF (2004). Seletividade de herbicidas aplicados em pré-emergência na cultura da cana-de-açúcar tratada com nematicidas. Planta Daninha 22:567-575.
Crossref |
|
|
|
Oliveira Júnior RS (2011). Mecanismos de Ação de Herbicidas. In: Oliveira Júnior R.S., Constantin J., Inoue M.H., editors. Biologia e manejo de plantas daninhas. Curitiba: Omnipax 141-192. |
|
|
|
Rede Oficial de Laboratórios de Análise de Solo e de Tecido Vegetal - ROLAS (2004) Manual de adubação e calagem para os estados do Rio Grande do Sul e Santa Catarina. Porto Alegre P. 400. |
|
|
|
Rodrigues BN, Almeida FS (2011). Guia de herbicidas. Sixth edition. Londrina P. 697. |
|
|
|
Secretaria de Estado da Agricultura e do Abastecimento - SEAB (2014). Produtos Florestais – Erva-mate. Curitiba 9p. |
|
|
Sharkey TD, Raschke K (1981). Effect of lightquality on stomatal opening in leaves of Xanthium strumarium L. Plant Physiol. 68:1170-1174.
Crossref |
|
|
Toledo REB, Victoria Filho R, Pitelli RA, Alves PLCA, Lopes MAF (2000). Efeito de períodos de controle de plantas daninhas sobre o desenvolvimento inicial de plantas de eucalipto. Planta Daninha 18:395-404.
Crossref |
|
|
Tuffi-Santos LD, Ferreira LR, Ferreira FA, Duarte WM, Tiburcio RAS, Machado AFL (2006). Intoxicação de eucalipto submetido à deriva simulada de diferentes herbicidas. Planta Daninha 24:521-526.
Crossref |
|
|
Vargas L, Roman, ES (2005). Seletividade e eficiência de herbicidas em cereais de inverno. Rev. Bras. Herb. 4:1-10.
Crossref |
|
|
Velini ED, Martins D, Manoel LA, Matsuoka S, Travain JC, Carvalho JC (2000). Avaliação da seletividade da mistura de oxyfluorfen e ametryne, aplicada em pré e pós-emergência, a dez variedades de cana-de-açúcar (cana planta). Planta Daninha 18:123-134.
Crossref |
|
|
|
Vidal RA, Merotto JA (2001). Herbicidologia. First edition. Porto Alegre: UFRGS P 152. |