ABSTRACT
Stalk rot of sorghum plants caused by Erwinia chrysanthemi is one of the most destructive diseases of sorghum crop. Twenty one of bacterial isolates were isolated from stalk of sorghum plants from different location of U.S. Nagar district of Uttarakhand, India. Biochemical, physiological and morphological characterization of E. chrysanthemi was done for confirmation of the bacterium specie. Bacterial suspension [0.7% Tween-40 (v/v) + 2 × 108 cell/ml] of each isolate was inject-inoculated with a 21G hypodermic needle into the stalk of plants for pathogenicity testing of the test bacterium. Reactions of biochemical and physiological testing are clearly evident enough to support the confirmation of test bacterium to taxonomic assignation of E. chrysanthemi causing soft roton sorghum plants. Out of 31 diseased samples of different locations examined, in 21 samples, the pathogen was detected as E. chrysanthemiby usingset of biochemical and physiological testing. As all the bacterium produced typical stalk rot disease symptoms on sorghum plant and water-insoluble blue pigment (indigoidine) on nutrient glycerol MnCl2.4H2O (2 mM) agar medium it was confirmed as chrysanthemi species.
Key words: Blue pigment, characterization, Erwinia chrysanthemi, Stalk rot, sorghum.
Soft rot erwinias are very important primary pathogens of both growing plants and the harvested crop (Pérombelon and Kelman, 1980). However, strains from different host plants differ in their specific host range as well as in the pathogenic and phenotypic properties (Dickey, 1979; Janse and Ruissen, 1988). The genus Erwinia is named after one of the founder of phytobacteriology, Erwin Frink Smith, and was established by Winslow et al. (1917) to include the plant pathogenic entereobacteria. Like other entereobacteria, the Erwinia are motile by means of several to many peritrichous flagella usually 8-11 (Burkholder et al., 1953; Dickey, 1981), gram-negative, non-spore forming, straight rod with rounded ends, and occurs singly or in pairs. Its size varies from 0.8-3.2 × 0.5-0.8 μm (average 1.8 × 0.6 μm) depending on carbon source present in the medium and growth conditions (Grula, 1970). Stalk rot of sorghum caused by Erwinia chrysanthemi Burkholder, McFadden, and Dimock is one of the most destructive diseases of sorghum crop. Saxenaet al. (1991) reported this bacterium causing stalk and top rot of sorghum under natural conditions in India during 1987-1988 crop season in sorghum field at Pantnagar, Uttarakhand. The disease was wide spread and affected 60 to 80% of plants in different sorghum genotypes. Recently, the occurrence of disease incidence ranging from 7.50 to 46.85 in Tarai region of Uttarakhand has been also shown by Kharayat and Singh (2013).
Early and accurate diagnoses of plant disease are necessary to predict outbreaks and allow time for development and application of mitigation strategies. A number of different biochemical methods are presently being employed for microbial identification and taxonomic classification. Moreover, each method has its advantages and disadvantages; with regard to ease of application, reproducibility, requirement for equipment and level of resolution. Present investigation was aimed to devise the set of biochemical testing to characterize the isolates of destructive soft rot bacterium, E. chrysanthemi.
On the basis of visual observation of suspected stalk with typical soft rot symptoms, sampling was done from 31 different locations in the growing season 2011-2012. Samples were brought to the laboratory and kept in refrigerator at 4°C. Isolations were made from samples and the pathogen was identified by using set of biochemical and physiological testing.
Isolations and purifications of E. chrysanthemi
Isolation of bacterium was done as per the method described by Janse (2005). Pieces of tissue taken from the margin of healthy and diseased tissues were disinfected with 70% alcohol and placed in a sealed tube with sterile water and tissues were left for 30 min in suspension so that the bacteria could diffuse out of the tissues. Subsequently 100 μl of the suspension was plated onto crystal violet sodium polypectate (CVP) medium. Characteristically deep-pit forming colony on CVP medium purified on yeast dextrose calcium carbonate medium by streaking using freshly growing single colony and these plates were incubated at 28°C for five days.
Pathogenicity tests
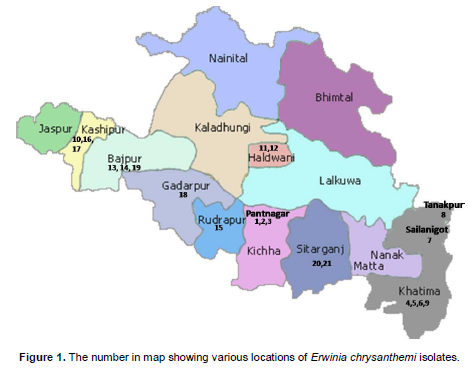
To confirm the pathogenicity of isolatesfrom various locations [Figure 1, 1-Pantnagar-1, 2-Pantnagar-2, 3-Pantnagar UTMC-535, 4-Majra Farm, 5-Banjari farm, 6-Chhinki Farm, 7-Sailanigot,8-Tanakpur,9-Khetalsanda,10-Kashipur,11-Haldhwani-1(HLD1),12-Kisanpur (Haldhwani), 13-Bajpur,14-Bajpur (Doraha),15-Rudrapur,16-Kashipur (Sultanpur),17-Tanda Kajal (Kashipur), 18-Gadarpur, 19-Barhani (Bajpur), 20-Sitarganj and 21-Nagina (Sitarganj)], stem inoculation was done on of 21 days old susceptible sweet sorghum plants variety SPSSV 6 under controlled glasshouse conditions. Isolates were grown on Luria Broth for 24 h at 28°C. The bacterial cells were suspended in sterile distilled water and the cell density adjusted to 2×108cfu/ml. Bacterial suspension [0.7 % Tween-40 (v/v) + 2 × 108 cell/ml] of each isolate was inject-inoculated with a 21G hypodermic needle into the stalk of plants. Control plants were inject-inoculated with sterilized water. Experiment was conducted twice to confirm the result.
Biochemical, physiological and morphological characterization of E. chrysanthemi
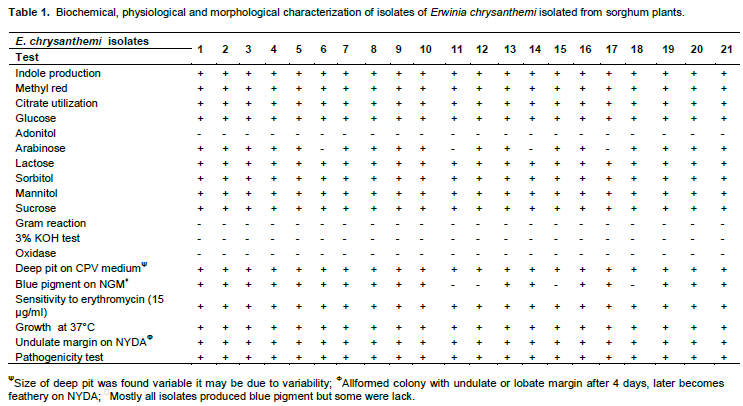
Test pathogen was screened for characterization upto species level by using a set of biochemical and physiological testing (Table 1) to detect the presumptive E. chrysanthemiwhich were selected according to keys of Schaad et al. (2001).
Scanning electron microscopy
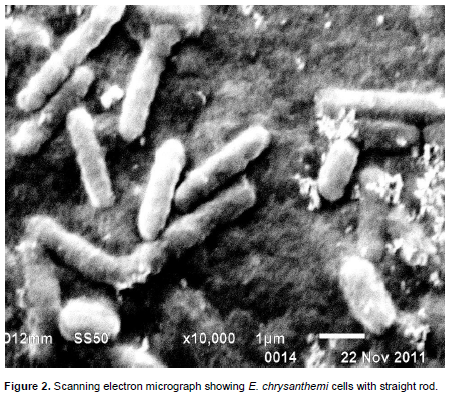
SEM preparation for E. chrysanthemi Pantnagar isolate was done using procedure described by Kaláb et al. (2008). Twenty-four hours old actively growing bacterial cells on Luria broth medium were harvested by centrifuge at 6000 rpm. Then, the bacterial cells were fixed with 2.5% gluteraldehyde in 0.05 M sodium phosphate, pH 6.8, for 24 h at 4ºC, washed with sodium cacodylate buffer three times (10 min each wash). They were finally fixed in 1% osmium tetraoxide for 1 h and washed with 0.1 M sodium cacodylate buffer as before. Further, the cells were dehydrated through a series of graded acetone (10, 20, 30, 40, 50, 70, 80, 90 and 100%). These cells were soaked for 15 min at each concentration. The drying was completed by placing the sample in a flow of CO2 in critical point dryer. The cells were mounted on aluminum stubs and coated with gold using Hummer V sputter coater, and viewed and photographed under a scanning electron microscope.
Reactions of biochemical and physiological testing presented in Table 1 are clearly evident enough to support the confirmation of test bacterium to taxonomic assignation of E. chrysanthemi causing soft roton sorghum plants. Out of 31 diseased samples of different locations (Figure 1) examined, in 21 samples, the pathogen was detected as E. chrysanthemi by using set of biochemical and physiological testing. These set of test also has been used by several other investigators to detect the presumptive E. chrysanthemi, viz. oxidative/fermentative test (Hugh and Leifson, 1953), oxidase test, deep pit formation on crystal violet sodium polypectate, CVP medium + 0.4% tetrazolium chloride solution (Cuppels and Kelman, 1974; Tomlinson and Cox, 1987; Perombelon and Burnett, 1991; Bdliyaet al., 2004; Kaneshiroet al., 2008; Zhu et al., 2010), indole production, sensitivity to erythromycin at 15 μg/ml (Jensen et al., 1986; Olabiyi, 2010), colony morphology on yeast dextrose calcium carbonate agar medium (YDC) (Dye, 1968; Goto, 1979; Kaneshiroet al., 2008), and Growth at 37°C (Pérombelon and Kelman, 1980; Lelliott and Dickey, 1984; Pérombelon and Hyman, 1986; Hyman et al., 1998). As the bacterium produced water-insoluble blue pigment (indigoidine) on NGM (Nutrient Glycerol MnCl2.4H2O (2 mM) agar medium itwas confirmed as chrysanthemi species. As it has been already reported that chrysanthemi is only species under the genus which produced water-insoluble blue pigment (Starr et al., 1966; Lee and Yu, 2006; Olabiyi, 2010). SEM analysis showed that the shape and size of bacterium, straight rod with rounded ends (Figure 2) and 1.50 × 0.50 μm respectively. In pathogenicity test, the bacterium found potential pathogen as it produced typical symptom on stalk of sorghum plant as naturally occurred in field conditions after 4 days of inoculation (Figure 3). The symptom mainly affect sorghum stem showing water-soaked symptoms that later turned reddish dark brown color. The infected stem pith disintegrated and showed slimy soft-rot symptoms after 7 days of inoculation. Several other workers also reported same symptoms (Zummo, 1969; Hepperly and Davila, 1987; Saxenaet al., 1991; Hseuet al., 2008).

Biochemical and physiological methods are easy to use, reproducible and less costly than molecular and serological methods and can be readily used for identification of E. chrysanthemi.
The authors have not declared any conflict of interest.
The authors thank Dr. Karuna Vishunavat, Head, Department of Plant Pathology, G.B. Pant University of Agriculture and Technology, Pantnagarfor providing necessary laband glasshouse facilities during the course of investigation.
REFERENCES
|
Bdliya BS, Lagerfeld E, Rudolph K (2004). A modified crystal violet pectate (CVP) medium for detection and isolation of soft rot Erwinia spp. from plant materials. J. Plant Dis. Prot. 111(5):506-515. |
|
|
|
Burkholder WH, McFadden LA, Dimock AW (1953). A bacterial blight of chrysanthemums. Phytopathology43:522-526. |
|
|
Cuppels D, Kelman A (1974). Evaluation of selective media for isolation of soft-rot bacteria from soil and plant tissue. Phytopathology 64:468-475.
Crossref |
|
|
|
Dickey RS (1979). Erwiniachrysanthemi: A comparative study of phenotypic properties of strains from several hosts and other Erwiniaspecies. Phytopathology69:324-329. |
|
|
|
Dickey RS (1981).Erwiniachrysanthemi: reaction of eight plant species to strains from several hosts and to strains of other Erwiniaspecies. Phytopathology71:23-29. |
|
|
|
Dye DW (1968). A taxonomic study of genus Erwinia I. The ''amylovora'' group. N. Z. J. Sci. 11:590-607. |
|
|
|
Goto M (1979). Bacterial foot rot disease of rice caused by a strain of Erwiniachrysanthemi. Phytopathology68:213-216. |
|
|
Grula MM (1970). Cell size of Erwinia sp. as influenced by composition of medium. Can. J. Microbiol.16(12):1363-1365.
Crossref |
|
|
|
Hepperly, Davila R (1987). Erwiniachrysanthemi Burk., McFaddanDimock: A bacterial whorl and stalk rot pathogen of sorghum [Sorghum bicolor (L.) Moench]. J. Agric.Univ. Puerto Rico. 71(3):265-275. |
|
|
|
Hugh R, Leifson E (1953). The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J. Bacteriol. 66:24-26. |
|
|
|
Hyman LJ, Toth IK,Perombelon MCM (1998). Isolation and identification. In: Perombelon MCM and Vander Wolf, JM (eds). Methods for the detection and quantification of Erwiniacarotovorapv. carotovora on potatoes. L. M. Invergowrie, Scottish Crop research Institute. pp. 60-65. |
|
|
Janse JD (2005). Phytobacteriology: Principles and Practices. CABI PublishingP. 366.
Crossref |
|
|
|
Janse JD, Ruissen MA (1988). Characterization and classification of Erwiniachrysanthemistrains from several hosts in the Netherlands.Phytopathology78:800-808. |
|
|
Jensen SC, Mayberry WR, Obrigawitch JA (1986). Identification of Erwiniachrysanthemias a soft rot-inducing pathogen of grain sorghum. Plant Dis.70:593-596.
Crossref |
|
|
|
Kaláb M, Yang AF, Chabot D (2008). Conventional Scanning Electron Microscopy of Bacteria. Infocuspp. 44-61. |
|
|
Kaneshiro WS, Burger M, Vine BG, de Silva AS, Alvarez AM (2008). Characterization of Erwiniachrysanthemifrom a bacterial heart rot of pineapple outbreak in Hawaii. Plant Dis. 92:1444-1450.
Crossref |
|
|
|
Kharayat BS, Singh Y (2013). Unusual occurrence of erwinia stalk rot of sorghum in tarai region of Uttarakhand. Int. J. Agric. Sci. 9(2):809-813. |
|
|
Lee YA, Yu CP (2006). A differential medium for the isolation and rapid identification of a plant soft rot pathogen, Erwiniachrysanthemi. J. Microbiol. Methods 64(2):200-206.
Crossref |
|
|
|
Lelliott RA, Dickey RS (1984). Genus VII. Erwinia. Bergey'smanual of systematic bacteriology, Williams and Wilkins, Baltimore, USA. pp.469-476. |
|
|
|
Olabiyi AM (2010). First report of Erwiniastem canker of papaya (Carica papaya L.) in Nigeria. Eur. J. Sci. Res. 46(3):422-430. |
|
|
PerombelonMCM, Burnett EM (1991). Two modified crystal violet pectate (CVP) media for the detection, isolation and enumeration of soft rot Erwinias. Potato Res. 34:79-85.
Crossref |
|
|
Pérombelon MCM, Hyman LJ (1986). A rapid method for identifying and quantifying soft rot erwinias directly from plant material based on their temperature tolerance and sensitivity to erythromycin. J. Appl. Bacteriol.60:61-66.
Crossref |
|
|
Pérombelon MCM, Kelman A (1980). Ecology of the soft rot erwinias. Ann. Rev. Phytopathol. 18:361-387.
Crossref |
|
|
|
Saxena SC, Mughogho LK, Pande S (1991). Stalk rot and top rot of sorghum caused by Erwiniachrysanthemi. Indian J. Microbiol. 31(4):435-441. |
|
|
|
Schaad NW, Jones JB, Chun W (2001).Laboratory guide for identification of plant pathogenic bacteria. APS Press, St. Paul, MN, USA. 158p. |
|
|
|
Starr MP, Cosens G, Knackmuss HJ (1966). Formation of the blue pigment indigoidine by phytopathogenicErwinia. Appl. Microbiol. 14:870-872. |
|
|
Tomlinson DL, Cox PG (1987). A new disease of cardamom (Elettariacardamomum) caused by Erwiniachrysanthemi in Papua New Guinea. Plant Pathol. 36(1):79-83.
Crossref |
|
|
|
Winslow CEA, Broadhurst J, Buchanan RE, Krumwiede CJ, Rogers LA, Smith GH (1917). The families and genera of the bacteria. Preliminary report of the committee of the society of American Bacteriologists on characterization and classification of bacterial types. J. Bacteriol. 2:505-566. |
|
|
|
Zhu L, Xie H, Chen S, Ma R (2010). Rapid isolation, identification and phylogenetic analysis of Pectobacteriumcarotovorum.J. Plant Pathol.92(2):479-483. |
|
|
|
Zummo N (1969). Bacterial soft rot, a new disease of sweet sorghum. Phytopathology 59:119. |