Full Length Research Paper
ABSTRACT
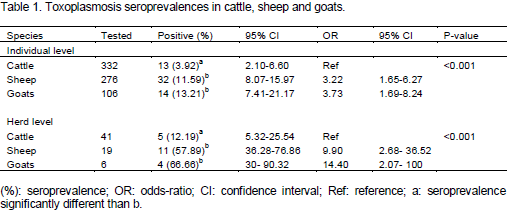
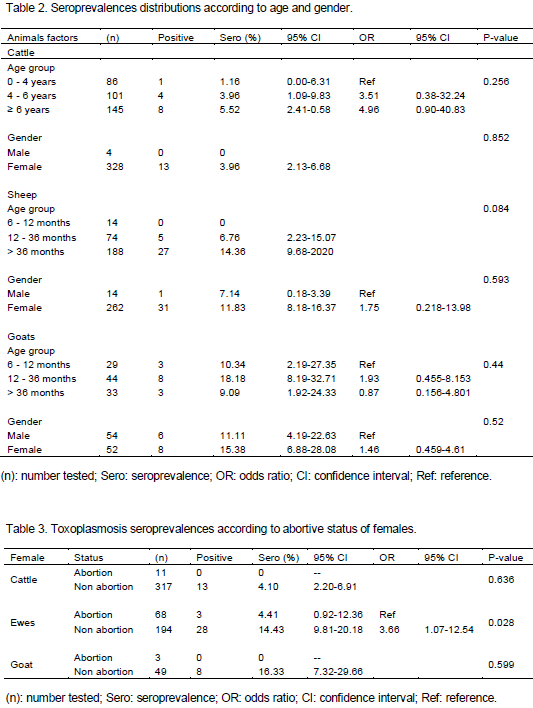
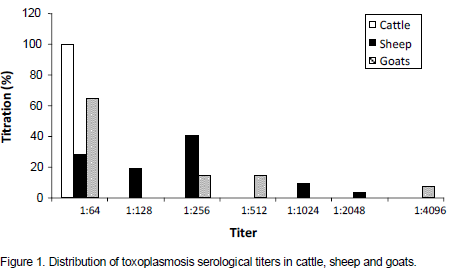
Toxoplasmosis is a parasitic disease with worldwide distribution and a major public health problem. In Algeria, human toxoplasmosis is screened in pregnant women and immunosuppressed persons; however, no information is available on the animal infection and a probable implication of the parasite in abortions occurring in the field. This sero-epidemiological cross-type survey on toxoplasmosis in cattle (332), sheep (276) and goats (106) revealed the presence of anti-Toxoplasma antibodies based on the indirect fluorescent antibody test (IFAT), at the respective rates of 3.92, 11.59 and 13.21%. The likelihood of acquiring Toxoplasma gondii infection was higher in sheep and goats (OR=3.22, 95% confidence interval [CI]: 1.65-6.27 and OR= 3.73, [CI]: 1.69-8.24 respectively) than in cattle (p<0.001). However, the difference between sheep and goats is not significant. At herd level, 5 herds out of 41 (12.19%), 11 herds out of 19 (57.89%) and 4 herds out of 6 (66.66%) showed at least one seropositive case in cattle, sheep and goat herds, respectively. Statistical comparison between genders and age groups showed no significant difference in the three species. The highest serological titers obtained are 1:64, 1:2048 and 1:4096 for cattle, sheep and goats, respectively. Suspicion of the parasite's role in abortions has been investigated, the seroprevalence showed no significant difference between abortive and non abortive females for cattle and goats; however, it was significantly higher in ewes that have not aborted as compared to those having abortions, a high suspicion was done for one abortive ewe whose antibody titer reached 1:1024. The presence of anti-Toxoplasma antibodies has been highlighted for the first time in livestock in Algeria, indicating a contamination with the parasite.
Key words: Toxoplasma gondii, seroprevalence, cattle, sheep, goats, abortion, Algeria.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSION
CONFLICT OF INTEREST
REFERENCES
|
Al-Mohammed HI (2011). Seroprevalence of Toxoplasma gondii Infection in Cats, Dogs and Ruminant Animals in Al-Ahsa Area in Saudi Arabia. Res. J. Med. Sci. 5:190-192. Crossref |
||||
| Bachi F, Gourbdji E, Taourirt L, Boudhane L, Lazizi L (2010). Toxoplasmose congénitale: bilan du centre national de référence (Algérie). Archives de l'institut Pasteur de Tunis. Tome 87:3-4. | ||||
|
Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Oréfice F, Addiss DG (2003). Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg. Infect. Dis. 9:55-62 Crossref |
||||
| Benkirane NJ, Rodolakis A (1990). Fréquence d'avortement et séroprévalence des principales maladies infectieuses abortives ovines dans la région de Rabat (Maroc). Ann. Rech. Vet. 21:267-273. | ||||
|
Bisson A, Maley S, Rubaire-Akiiki CM, Wastling JM (2000). The seroprevalence of antibodies to Toxoplasma gondii in domestic goats in Uganda. Acta Tropica. 76:33-38. Crossref |
||||
|
Blewett DA, Watson WA (1984). The epidemiology of ovine toxoplasmosis. III. Observations on outbreaks of clinical toxoplasmosis in relation to possible mechanisms of transmission. Br. Vet. J. 140:54–63. Crossref |
||||
|
Boughattas S, Ayari K, Sa T, Aoun K, Bouratbine A (2014). Survey of the Parasite Toxoplasma gondii in Human Consumed Ovine Meat in Tunis City. PLoS One 9(1):1-5. Crossref |
||||
|
Camossi LG, Greca-Júnior H, Corrêa AP, Richini-Pereira VB, Silva RC, Da Silva AV, Langoni H (2011). Detection of Toxoplasma gondii DNA in the milk of naturally infected ewes. Vet. Parasitol. 177:256-261. Crossref |
||||
| Carneiro ACAV, Carneiro M, Gouveia AMG, Vilas-Boas LS, Vitor RWA (2009). Seroprevalence and risk factors of sheep toxoplasmosis in Minas Gerais, Brazil. Revue Méd. Vét. 160(11):527-531. | ||||
|
Clementino MM, Souza MF, Andrade Neto VF (2007). Seroprevalence and Toxoplasma gondii-IgG avidity in sheep from Lajes, Brazil. Vet. Parasitol.146:199-203. Crossref |
||||
|
Cook AJC, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, Foulon W, Semprini AE, Dunn DT (2000). Sources of Toxoplasma infection in pregnant women: european multicentre case-control study. Br. Med. J. 321:142–147. Crossref |
||||
|
de Moura L, Bahia-Oliveira LM, Wada MY, Jones JL, Tuboi SH, Carmo EH, Ramalho WM, Camargo NJ, Trevisan R, Graca RM, da Silva AJ, Moura I, Dubey JP, Garrett DO (2006).Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 12:326–329. Crossref |
||||
| Dechicha A, Gharbi S, Kebbal S, Chatagnon G, Tainturier D, Ouzrout R, Guetarni D (2010). Serological survey of etiological agents associated with abortion in two Algerian dairy cattle breeding farms. J. Vet. Med. Anim. Health 2(1):001-005. | ||||
|
Dubey JP, Frenkel JK (1972). Cyst-induced toxoplasmosis in cats. J. Protozool. 19:155–177. Crossref |
||||
|
Dubey JP (1986). A review of toxoplasmosis in cattle. Vet. Parasitol. 22: 177-202. Crossref |
||||
| Dubey JP, Beattie CP (1988). Toxoplasmosis of Animals and Man. CRC Press, Boca Raton, FL. P. 200. | ||||
|
Dubey JP, Kirkbride CA (1989). Enzootic toxoplasmosis in sheep in North-Central United-States. J. Parasitol. 75:673-676. Crossref |
||||
| Dubey JP, Lunney JK, Shen SK, Kwok OCH, Ashford DA, Thulliez P (1996). Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J. Parasitol. 82:438–443. | ||||
|
Dubey JP (2004) Toxoplasmosis – a waterborne zoonosis. Vet. Parasitol.126:57–72. Crossref |
||||
|
Dubey JP, Jones JL (2008). Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38:1257–127. Crossref |
||||
|
Dumetre A, Ajzenberg D, Rozette L, Mercier A, Dardé ML (2006). Toxoplasma gondii infection in sheep from Haute-Vienne, France: Seroprevalence and isolate genotyping by microsatellite analysis. Vet. Parasitol. 142:376-379. Crossref |
||||
|
El fahal AM, Elhassan AM, Hussien MO, Enan KA, Musa AB, El Hussein AM (2013). Seroprevalence of Toxoplasma gondii in Dairy Cattle with Reproductive Problems in Sudan. ISRN Vet. Sci. 1-4. Crossref |
||||
| EU (2010). Directive on the protection of animals used for scientific purposes. | ||||
|
Evengard B, Petersson K, Engman ML, Wiklund S, Ivarsson SA, Tear-Fahnehjelm K, Forsgren M, Gilbert R, Malm G (2001). Low incidence of toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiol Infect.127: 121-127. Crossref |
||||
| Figueiredo JF, Silva DAO, Cabral DD, Mineo JR (2001). Seroprevalence of Toxoplasma gondii Infection in Goats by the Indirect Haemagglutination, Immunofluorescence and mmunoenzymatic Tests in the Region of Uberlândia, Brazil. Mem Inst Oswaldo Cruz, Rio de Janeiro. 96(5):687-692. | ||||
|
Garcia JL, Navarro IT, Ogawa L, Claret de Oliveira R (1999). Seroprevalência do toxoplasma gondii, em suínos, bovinos, ovinos e eqüinos, e sua correlação com humanos, felinos e caninos, oriundos de propriedades rurais do norte do paraná-brasil. Ciência Rural, Santa Maria. 29(1):91-97. Crossref |
||||
|
Gebremedhin EZ, Abdurahaman M, Hadush T, Tessema TS (2014). Seroprevalence and risk factors of Toxoplasma gondii infection in sheep and goats slaughtered for human consumption in Central Ethiopia. BMC Research Notes. 7:696. Crossref |
||||
|
Halos L, Thébault A, Aubert D, Thomas M, Perret C, Geers R, Alliot A, Escotte-Binet S, Ajzenberg D, Dardé ML, Durand B, Boireau P, Villen I (2010). An innovative survey underlining the significant level of contamination by Toxoplasma gondii of ovine meat consumed in France. Int. J. Parasitol. 40:193-200. Crossref |
||||
| Hamzy El Idrissi A, Manyari A, Benkirane A (1995). Fréquence des avortements infectieux des ovins au Maroc (régions des Zaer et du Moyen Atlas). Actes Inst. Agron. Veto (Maroc). 15(4):11-14. | ||||
| Holec-GÄ…sior L, DrapaÅ‚a D, Dominiak-Górski B, Kur J (2013). Epidemiological study of Toxoplasma gondii infection among cattle in Northern Poland. Ann. Agric. Environ. Med. 20(4|):653–656. | ||||
|
Jackson MH, Hutchison WM (1989). The prevalence and source of Toxoplasma infection in the environment. Adv. Parasitol.28:55-105. Crossref |
||||
|
Jenum PA, Kapperud G, Stray PB, Melby KK, Eskild A, Eng J (1998). Prevalence of Toxoplasma gondii specific immunoglobulin G antibodies among pregnant women in Norway. Epidemiol. Infect.120:8792. Crossref |
||||
|
Jones JL, Lopez B, Alvarez Mury M, Wilson M, Klein R, Luby S, Maguire JH (2005). Toxoplasma gondii infection in rural Guatemalan children. Am. J. Trop. Med. Hyg. 72:295–300. PMid:15772325 |
||||
|
Kamani J, Mani AU, Egwu GO (2010). Seroprevalence of Toxoplasma gondii infection in domestic sheep and goats in Borno state, Nigeria. Trop Anim Health Prod. 42(4):793-797. Crossref |
||||
|
Khezri M, Mohammadian B, Esmailnia K, Khezri O (2012). Toxoplasmosis in sheep from Kurdistan province, Iran. Afr. J. Microbiol. Res. 6(18):3989-3992. Crossref |
||||
|
Klun I, Djurkovic´-Djakovic´ O, Katic´-Radivojevic´ S, Nikolic´A (2006). Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep and pigs in Serbia: Seroprevalence and risk factors. Vet. Parasitol. 135:121-131. Crossref |
||||
| Lafi SQ, Giadinis ND, Papadopoulos E, Filioussis G, Koutsoumpas A (2014). Ovine and caprine toxoplasmosis: experimental study. Pak. Vet J. 34(1): 50-53. | ||||
| Lindsay DS, Dubey JP (2007). Toxoplasmosis in wild and domestic animals L.M. Weiss, K. Kami (Eds.), Toxoplasma gondii. The Model Apicomplexan: Perspectives and Methods, Academic Press, London, UK. pp. 133–152. | ||||
|
Luciano DM, Menezes RC, Ferreira LC, Nicolau JL, Das Neves LB, Luciano RM, Dahroug MAA, Amendoeira MRR (2011). Occurrence of anti-Toxoplasma gondii antibodies in cattle and pigs slaughtered, State of Rio de Janeiro. Rev. Bras. Parasitol. Vet. Jaboticabal. 20(4):351-353. Crossref |
||||
|
Mahboub HD, Helal MA, Abd Eldaim MA, Abd El-Razek EM, Elsify AM (2013). Seroprevalence of abortion causing agents in egyptian sheep and goat breeds and their effects on the animal's performance. J. Agric. Sci. 5(9):92-101. Crossref |
||||
| Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV (1999) Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–624. | ||||
|
Messerer L, Bouzbid S, Gourbdji E, Mansouri R, Bachi F (2014). Séroprévalence de la toxoplasmose chez les femmes enceintes dans la wilaya d'Annaba, Algérie Revue d'Épidémiologie et de Santé Publique. 62(2):160–165. Crossref |
||||
|
Nematollahi A, Moghddam G (2008). Survey on Seroprevalence of Anti-Toxoplasma gondii Antibodies in Cattle in Tabriz (Iran) by IFAT. Am. J. Anim. Vet. Sci. 3(1):40-42. Crossref |
||||
|
Neto JOA, Azevedo SS, Gennari SM, Funada MR, Pena HFJ, Arau’jo ARCP, Batista BSA, Silva MLCR, Gomes AAB, Piatti RM, Alves CJ (2008). Prevalence and risk factors for anti-Toxoplasma gondii antibodies in goats of the Serido´ Oriental microregion, Rio Grande do Norte state, Northeast region of Brazil. Vet. Parasitol. 156:329-332. Crossref |
||||
| OIE (2008). Manuel terrestre de l'OIE. | ||||
|
Opsteegh M, Teunis P, Mensink M, Zuchner L, Titilincu A, Langelaar M,van der Giessen J (2010). Evaluation of ELISA test characteristics and estimation of Toxoplasma gondii seroprevalence in Dutch sheep using mixture models. Prev. Vet. Med. 96:232–240. Crossref |
||||
|
Pita Gondim LF, Barbosa Jr HV, Ribeiro Filho CHA, Saeki H (1999). Serological survey of antibodies to Toxoplasma gondii in goats, sheep, cattle and water buffaloes in Bahia State, Brazil. Vet. Parasitol. 82:273–276. Crossref |
||||
| Raeghi S, Akaberi A, Sedeghi S (2011). Seroprevalence of Toxoplasma gondii in Sheep, Cattle and Horses in Urmia North-West of Iran. Iran J. Parasitol. 6(4):90-94. | ||||
|
Roberts T, Murrell KD, Marks S (1994). Economic losses caused by foodborne parasitic diseases. Parasitol. Today. 10:419–423. Crossref |
||||
|
Rossi GF, Cabral DD, Ribeiro DP, Pajuaba AC, Corrêa RR, Moreira RQ, Mineo TW, Mineo JR, Silva DA (2011). Evaluation of Toxoplasma gondii and Neospora caninum infections in sheep from Uberlândia, Minas Gerais State, Brazil, by different serological methods. Vet Parasitol. 75:252-259. Crossref |
||||
| Rozette L, Dumètre A, Couquet CY, Dardé ML (2005). Séroprevalence de la toxoplasmose chez des ovins et des bovins en Haute-Vienne. Epidémiol. Santé Anim. 48:97-99. | ||||
| Ruiz A, Frenkel JK (1980). Toxoplasma gondii in Costa Rican cats. Am. J. Trop. Med. Hyg. 29: 1150–1160. PMID: 7446806. | ||||
|
Santos LM, Damé MC, Cademartori BG, da Cunha Filho NA, Farias NA, Ruas JL (2013). Occurrence of antibodies to Toxoplasma gondii in water buffaloes and meat cattle in Rio Grande do Sul State, southern Brazil. Acta Parasitol. 58(3):334-336. Crossref |
||||
| Sevgili M, Babur C, Nalbantoglu S, Karas G, Vatansever D (2005). Determination of Seropositivity for Toxoplasma gondii in Sheep in Sanliurfa Province. Turk. J. Vet. Anim. Sci. 29:107-111. | ||||
|
Sharif M, Gholami SH, Ziaei H, Daryani A, Laktarashi B, Ziapour SP, Rafiei A, Vahedi M (2007). Seroprevalence of Toxoplasma gondii in cattle, sheep and goats slaughtered for food in Mazandaran province, Iran, during 2005. Vet. J. 174:422-424. Crossref |
||||
| Smith JL (1993). Documented outbreaks of toxoplasmosis. Transmission of Toxoplasma gondii to humans. J. Food Protect. 56:630-639. | ||||
|
Spalding SM, Amendoeira MRR, Klein CH, Ribeiro LC (2005). Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev. Soc. Bras. Med. Trop. 38(2):173-177. Crossref |
||||
|
Swai ES, Kaaya JE (2012). A survey of Toxoplasma gondii antibodies by latex agglutination assay in dairy goats in Northern Tanzania. Trop. Anim. Health Prod. 45(1):211-217. Crossref |
||||
|
Tenter AM, Heckeroth AR, Weiss LM (2000). Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217–1258. Crossref |
||||
|
Vesco G, Buffolano W, La-Chiusa S, Mancuso G, Caracappa S, Chianca A, Villari S, Curro V, Liga F, Petersen E (2007). Toxoplasma gondii infections in sheep in Sicily, Southern Italy. Vet. Parasitol. 146:199-203. Crossref |
||||
|
Zewdu E, Agonafir A, Tessema TS, Tilahun G, Medhin G, Vitale M, Di Marco V, Cox E, Vercruysse J, Dorny P (2013). Seroepidemiological study of caprine toxoplasmosis in East and West Shewa Zones, Oromia Regional State, Central Ethiopia. Res. Vet. Sci. 94(1):43-48. Crossref |
||||
|
Zhao GH, Zhang MT, Lei LH, Shang CC, Cao DY, Tian TT, Li J, Xu JY, Yao YL, Chen DK, Zhu XQ (2011). Seroprevalence of Toxoplasma gondii infection in dairy goats in Shaanxi Province, Northwestern China. Parasites Vectors. 4:47. Crossref |
||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0