ABSTRACT
Agronomic performances of local and exotic Musa species were evaluated across seven agro-ecologies in Eastern Democratic Republic of Congo. Generally, all the cultivars performed well. Mean bunch yields varied between 11 and 42 kg across different use groups and agro-ecologies. Introduced cultivars had higher or comparable yields to the local cultivars across agro-ecologies. The exotic beer cultivar ‘NARI 27’ generally outperformed other beer types. The green cooking exotic types ‘NARITA 4’ and ‘NARITA 2’ produced bunches of up to 37 and 39 kg, respectively at altitudes of 1066 and 1111 m, though having smaller bunches of 21 to 25 kg, at 900 and 1707 m in comparison to the local type ‘Barhabeshya’ (30 to 37 kg). Yields were mainly influenced by soil factors and altitude. Yields generally increased with increase in OM, N, P, K, Ca, and pH. Altitude had a non-linear relationship with the time from planting to flowering, with the time from planting to flowering declining at higher altitudes. In contrast, the fruit filling phase increased linearly with altitude. Bunch weights of most cultivars declined with increasing altitude; particularly, when N, K, P and OM concentrations were low; possibly because most assimilates go towards sucker development at the high altitudes. For example, bunch weights of ‘Barhabeshya’, ‘Mbwazirume’ and ‘Nshika’ strongly declined (R2 = -0.56-0.99) with increasing altitude. ‘Ndundu’, ‘FHIA21’ and ‘Gros Michel’ thrived well at high altitude sites. Such variations in cultivar adaptability plus cultivar attributes, e.g. height can be exploited by selectively promoting cultivars in specific agro-ecologies/niches.
Key word: Agro-ecologies, cultivar, fruit filling phase, Kivu provinces, plantain cultivar, yield.
Plantain (Musa AAB), as well as other cooking banana (Musa ABB), East Africa highland banana (Musa AAA-EA) and dessert banana (Musa AAA, AA), constitute the sixth most important global food commodity (FAOSTAT, 2015). They are an excellent food source and in some countries of the world (e.g. in parts of East and Central Africa) they are the principal components of the diet. The all year round fruiting habit of banana puts the crop in a superior position in bridging the ‘hunger gap’ between annual crop harvests (INIBAP, 1996); therefore, contributing significantly to food and income security of people engaged in its production and trade, particularly, in developing countries. Moreover, banana is considered an important food, because of its chemical composition and high content of vitamins and minerals, particularly potassium (Silva et al., 2002). The pseudostems and leaves are commonly used as mulch in plantations, livestock feed and as wrapping material (Karamura, 1993). Dried leaf bases are extensively used as roofing material for houses, to weave ropes for tethering goats and sheep, and for mattress making (Karamura, 1993; Kamira et al., 2015). In Eastern and Central Africa, banana is commonly grown by small-scale farmers (Bagamba et al., 1999) whose farm size ranges from 0.5 to 1.5 ha. In East and Central Africa banana is mainly cultivated in association with other crops (Nyabyenda, 2006) and its importance is exemplified by the large proportion of land allocated to the crop (Bagamba et al., 1999).
A wide variety of Musa genome groups are cultivated worldwide (Pollefeys et al., 2004). This diversity manifests itself in the cultivars that are grown, the way they are prepared, eaten and marketed, and in the systems in which they are produced. Relatively few banana cultivars have moved from the center of origin in Southeast Asia, with the result that the diversity of these plants declines from Asia to Africa to America (Simmonds, 1995). The Great Lakes region covering parts of Uganda, Rwanda, Burundi, Tanzania, Kenya and Democratic Republic of Congo (DR Congo) is regarded as a secondary centre of diversity for bananas and plantains (Simmonds, 1966). This region is also the largest producer and consumer of bananas in Africa (Smales, 2006), with annual per capita consumption reaching 250 kg, the highest in the world (FAO, 1985).
The three major genomic groups under cultivation worldwide are AAA, AAB, and ABB (Simmonds, 1995). Most of the commercial cultivars are triploids and belong to the AAA dessert group. The cultivars grown vary with altitude. For instance, at lower elevations in the Eastern DR Congo and Congo basin, below 1,200 metres above sea level (masl), AAB plantains are mainly cultivated (Dheda et al., 2011; Ocimati et al., 2013). In contrast, in the mid to high altitude regions of Eastern DR Congo (1,200 to 2,000 masl) the East African highland banana types (AAA-EA) predominate (Ocimati et al., 2013).
However, above 2,000 masl, the majority of Musa cultivars do not perform well due to the low temperature (Turner et al., 2007; Sikyolo et al. 2013).
Banana production in Central Africa is mainly hampered by pests, including nematodes and weevils, as well as diseases (e.g. Xanthomonas wilt of banana [XW], banana bunchy top disease and Fusarium wilt), lack of resistant cultivars, poor soil fertility, and plantation management (Gold et al., 1994; INIBAP, 2003; Speijer et al., 1999; van Asten et al., 2011).
All the important nematode species that feed on bananas are root parasites which cause lesions, thereby reducing water and nutrient uptake to the upper parts of the plant and also paving the way for other pathogenic micro-organisms (Coyne et al., 2003) to infect plants. Nematodes are found on all varieties of bananas but variation in susceptibility has been observed (Gowen, 1995; Speijer, 1996; Speijer et al., 1999; Kamira et al., 2013). Banana weevils are especially important at the low altitude areas. Weevil larvae primarily destroy the rhizome tissue by tunneling through it as they feed (Gold et al., 2001). Severe weevil damage in the corm leads to a reduction in plant growth and bunch size, and to possible corm snapping due to wind or the weight of the plant (Gold et al., 1994). The weevil has been implicated in the decline and disappearance of highland banana from traditional growing zones in East Africa (Gold et al., 1999).
Banana Xanthomonas wilt is a devastating disease caused by the bacterium Xanthomonas campestris pv. musacearum. It was first officially reported in 1968 in Ethiopia (Yirgou and Bradbury, 1974), where it remained confined until 2001 when the disease appeared in both Central Uganda and the North Kivu province of the DR Congo and all banana cultivars planted in these areas are susceptible to XW (Tushemereirwe et al., 2004; Ndungo et al., 2006). Black leaf streak (BLS), the most important foliar disease in banana, caused by an airborne fungus called Mycosphaerella fijiensis Morelet, reduces functional leaf area and thus can cause yield losses of 30 to 50% (Mobambo et al., 1996; Ploetz, 2004). The majority of exotic bananas, together with all the East African highland bananas, are susceptible to BLS.
One pillar of the Consortium for Improving Agriculture-based Livelihoods in Central Africa (CIALCA)’s intervention strategy in banana-based systems is the introduction of new high yielding, pest and disease resistant Musa cultivars that are highly acceptable to consumers (CIALCA, 2008). Increasing the diversity of Musa germplasm forms an integral part of technology packages intended to overcome a number of biotic and abiotic challenges that hamper banana production in central Africa, a region where few exotic Musa cultivars have been introduced over the past decades.
In this study, the agronomic performance of introduced (exotic landraces and hybrids) plantain, green cooking, dessert and beer cultivars was evaluated against plantains, green cooking and beer landrace cultivars in three sites across South Kivu and four sites across North Kivu in the eastern DR Congo. The introduced cultivars were selected for a range of attributes that include one or more of the following: tolerance to pests and diseases, large bunch size, short to intermediate growth cycle, and ability to serve multiple purposes. Of particular interest was the association between agronomic features of cultivars with the altitude and soil conditions.
Musa germplasm experiments were established in 2007 in different agro-ecological zones with contrasting altitude, soil fertility and rainfall across the Kivu provinces of eastern DR Congo (Table 1 and 2). These included four sites in North Kivu (Butembo, Maboya, Mavivi and Mutwanga) and three sites in South Kivu (Kamanyola, INERA-Mulungu and Mushweshwe).


Exotic plantain cultivars (‘FHIA21’, ‘Obubit’, ‘T6’), green cooking (‘NARITA 2’ (previously ‘NSH20’), ‘NARITA 4’ (previously ‘NSH22’), ‘Mpologoma’), dessert (‘FHIA03’), and a beer cultivar (NARITA 27 (previously ‘NSH42’)) were planted and evaluated against the local plantain (‘Musheba’), local green cooking (‘Barhabeshya’, ‘Matooke’, ‘Mukingiro’, ‘Vulambya’, ‘Mbwazirume’), local beer (‘Ndundu’, ‘Nshika’) and dessert (‘Gros Michel’, ‘Dwarf Cavendish’, ‘Giant Cavendish’) cultivars (Table 3). The term ‘exotic’ is used in this text to refer to plantain and banana hybrids and landraces introduced from regions other than the study locations. The cultivars differ in their growth characteristics, tolerance or resistance to the various
Musa pests and diseases (Table 3). The exotic materials from the Bioversity International Transit Centre (ITC) in Leuven, Belgium were multiplied at the Agrobiotech tissue culture lab in Bujumbura, Burundi, while the IITA/NARO hybrids were multiplied at the Phytolabu lab in Bujumbura.
Sword suckers of the local cultivars that served as checks were obtained from the Institut National pour l’Étude et la Recherche Agronomiques (INERA) Mulungu, South Kivu Musa germplasm collection (Table 3), while the highland cooking banana ‘Mplologoma’ was obtained from Rwanda. Sucker planting material was pared and a 30 cm section of the pseudostem was left on the corm.
Suckers were planted with a small portion (15 cm) of the pseudostem sticking out of the ground. Hardening of tissue culture (TC) plantlets was done at the INERA Mulungu research station in South Kivu, DR Congo. Three month old TC-derived plantlets (on average 30 cm high) were transported to the various sites in South and North Kivu for subsequent planting.
Fifteen plants of each cultivar (in three replicates of five plants) were planted at each experimental site. Plants were spaced at 3 × 2 m, providing a density of 1,667 plants/ha. The size of the planting hole was 60 × 60 × 60 cm and 10 kg of decomposed cow manure was applied in each planting hole at planting. Weeding was carried out at three-monthly intervals, while de-suckering and de-leafing of dead leaves was practiced on a case-by-case basis. Three plants were kept per mat (that is, parent, first ratoon and second ratoon). Mulching was carried out at the beginning of each dry season. Where necessary, forked wooden sticks were used to support mature plants with heavy bunches to prevent toppling. The geographical coordinates of each experimental site were recorded using a GARMIN Global Positioning System (GPS) unit (Table 1). In addition, composite soil samples (0 to 30 cm soil layer) were collected at each location to determine soil physical and chemical characteristics (Table 2). Rainfall data (Table 1) were derived from radar images and were provided by the International Center for Tropical Agriculture’s Climate Change, Agriculture, Food Security programme.
Banana growth and yield data were collected and averaged over three cropping cycles (that is,plant crop cycle, 1st ratoon and 2nd ratoon). Data collected during each cycle comprised plant performance indicators at flowering (plant height, pseudostem circumference at soil level and at one metre above soil level and number of functional leaves) and at harvest (bunch weight, number of fruits per bunch, average number of fruits per hand, number of hands per bunch, average fruit length of the second lowest hand) and number of days from planting to flowering and from planting to harvest. Plant height was measured from soil level to the point where the leaf petioles of the youngest two leaves intersect, while the total number of functional leaves was determined by counting all the existing green leaves on a plant. The functional leaves had at least 50% green leaf lamina surface area. Mature bunches were harvested when the fingers of the second lowest hand had attained a round shape (Nguthi et al., 1999). Bunch weight was measured with a spring balance. Average annual production was calculated using the formula described by Gaidashova et al. (2008) as:
Average annual production = Bunch weight/number of days to harvest × 365 × plant density ha-1 where 365 is the number of days in a year.
Statistical analysis was carried out using Statistics Analysis System (SAS) (SAS Institute Inc., 2008). The General Linear Model procedure was used to analyze the data and the Tukey’s student range test was used for multiple comparisons. Averages for various growth and yield traits were computed across the three cropping cycles. Soil, weather and plant growth and yield attributes across the cultivars were subjected to a principal component analysis (PCA) using GenStat statistical software (GenStat, 2008) to determine the most important variables that influenced bunch yield (kg), time to flowering (days), time from flowering to harvest (days) and production (t ha-1 year-1). To further our understanding of the effect of altitude, association plots between altitude and bunch weight, time from planting to flowering, time from flowering to harvest, and total production were developed.
Yield across banana use groups and sites was affected by a combination of factors that included soil characteristics, altitude and plant attributes (Table 1 and 4, Figures 1 to 5).
Significant variations (P < 0.05) in agronomic performance within plantain cultivars (AAB and AAAB genomes), green cooking (AAA-EA), dessert (AAA and AABB) and beer (AAA-EA and other AAA) banana cultivars was observed within and across sites for plant height, pseudostem girth, number of leaves, crop cycle duration and all yield parameters (Table 5 to 9).
The effect of altitude, soil and plant characteristics on plant performance and yield
A principle components analysis to determine the contribution of altitude, soil and plant characteristics on plant performance and yield revealed that soil characteristics had the highest contribution to the variation in banana yields (Table 4) across sites. The first, second and third PCs had eigenvalues > 1 and contributed 35, 28 and 18%, respectively to the total variation in the data set while combined together they accounted for 81% of the total variation. PC 1 was mainly influenced by organic matter and soil macro-nutrient content, that is, phosphorus, potassium and nitrogen levels. PC 2 was mainly influenced by soil pH, calcium and magnesium. PC 3 was explained by the yield attribute, the number of hands on a bunch and the growth characteristics (that is, plant girth and height) of the banana cultivars. Altitude (PC 2) contrasted with soil fertility content, pH and the banana growth and yield attributes (Table 4).

The principal component plot for bunch weight separated each location, with Mulungu showing the strongest positive association with K, N, OM and P concentrations in the soil and bunch weight. In soils with lower concentrations of K, N, P and OM, the association between bunch weight and Ca, Mg and pH became more apparent. Altitude had a negative association with bunch weight, particularly when N, K, P and OM concentrations are low. Within each site there is a slope of the plots towards the upper left section of the graph (Figure 1). This may reflect the consistent effect of soil fertility across the range of genotypes at each site.

The principal component plot for time from planting to bunch emergence divides the sites into five groups. Mulungu has a high association between time from planting to bunch emergence and soil N, P, K, and OM concentrations. Mavivi falls low on the PC1 axis with expected low concentrations of N, P, K and OM, but with large plants, and these features are associated with an increased time from planting to bunch emergence and low suckering. Maboya had a strong association with soil pH, Ca and Mg. The fourth group of sites straddles the PC1 axis close to the origin, but is spread along the PC2 axis. Here at moderate fertility the stronger associations between plant size (girth and height) and time from planting to bunch emergence appear.
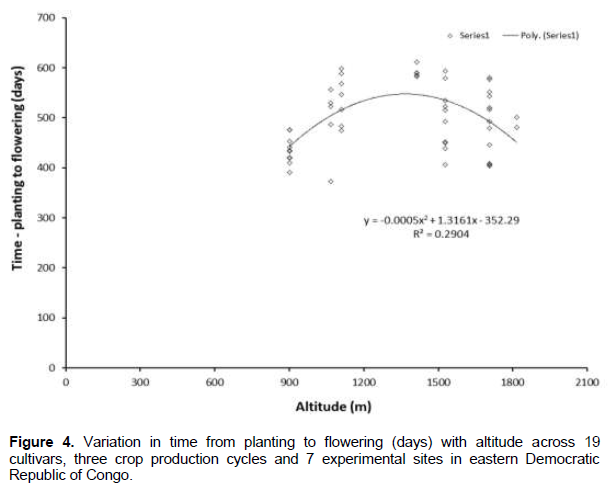
Altitude did not significantly influence the time from planting to bunch emergence, while it influenced bunchweight and production (Figures 2 and 3). Since higher altitudes are associated with cooler temperatures that slow the development of bananas, one would expect an association between altitude and time from planting to bunch emergence to be present in the PCA. A plot of the time from planting to bunch emergence against altitude (using data from Tables 5, 7, 8 and 9) resulted in variable data and the linear regression gave an R2 of 0.045, indicating no association. However, when a polynomial line was fitted to the data the R2 increased to 0.29 (Figure 4).


To further evaluate the association between altitude and development, altitude was plotted against the time from bunch emergence to harvest. As expected, and in line with for example Sikyolo et al. (2013), an increased fruit filling phase was observed with increasing altitude (Figure 5), with an R2 of 0.30 for a polynomial function. The fruit filling phase is likely to be largely free of the effect of early or late suckering that is likely to influence the data on planting to bunch emergence.
Performance of exotic and local plantain cultivars
The cultivar ‘T6’ had the highest mean plant height (316 cm) and girth (75 cm), while ‘FHIA21’ had the smallest plant height (287 cm) and girth (69 cm) across the sites. Altitude did not have a consistent effect on plant height or girth. In general, the cultivar ‘Musheba’ had the highest mean number of functional leaves (8.8 leaves), while the least were noted in ‘Obubit’ (Table 5). The highest number of functional leaves was recorded at Kamanyola, that has lowest altitude (900 masl), although no consistent trend was observed with increasing altitude. Generally the cultivars took longer to mature at the high altitude sites. For example the fastest developing plantain cultivar was ‘T6’ recorded at the low altitude site at Mavivi (1066 masl; flowering at 373 days and harvested at 502 days) followed by ‘Musheba’ at Kamanyola (900 masl; 546 days to harvest). ‘Obubit’ and ‘FHIA21’, respectively had the longest time to flowering at the high and mid altitude sites at Mulungu (1707 masl; 580 days) and Mushweshwe (1528 m; 579 days). The mid altitude sites of Maboya (1412 masl) and Mushweshwe, characterized by high erosion and poorer soils (Table 2), generally recorded long crop cycles. For example, ‘T6’ and ‘FHIA21’ took the longest time from planting to harvest at Maboya (727days) and Mushweshwe (741days), respectively (Table 5).



Performance of local/regional and hybrid green cooking banana cultivars
Among the green cooking cultivars, ‘Barhabeshya’ (359 cm) recorded the highest plant height, though ‘Mukingiro’ (415 cm) and ‘NARITA 2’ (408 cm) had above average heights at Butembo (1815 masl) and Mavivi (1066 m), respectively. ‘Matooke’ (231 cm) was the shortest cultivar (Table 7). Plant height generally increased with increasing altitude across all the cultivars evaluated except for ‘Mpologoma’ (Table 6). However, significant increases in plant height were only observed in ‘Barhabeshya’ (R2=0.96) and ‘Mbwazirume’ (R2=0.67). The largest mean pseudostem girth at soil level was recorded for ‘Barhabeshya’ (83 cm) followed by ‘Mbwazirume’ (82 cm) and the least in ‘Matooke’ (71 cm) (Table 7). Except for ‘Mbwazirume’ (R2=0.86), plant girth generally increased with altitude (Table 6).
On average, the introduced cultivar ‘Mpologoma’ (11.3 leaves) had the highest mean number of functional leaves at flowering followed by ‘Barhabeshya’ (11.0 leaves) and the least in‘Mukingiro’ (6.4 leaves).
Mushweshwe (mid altitude) followed by Kamanyola (low) generally had the highest number of leaves (Table 7). Apart from ‘Matooke’ whose leaves increased with altitude (R2=0.42) and 'NARITA 4' whose number of leaves profoundly declined with altitude (R2=-0.33), leaves in other cultivars were not significantly impacted impacted by the changes in altitude (Table 6).Generally the time to harvest increased with altitude except for ‘Mpologoma’ (Tables 6 and 7). The lowest time to maturity was generally recorded at the lowest altitude site in Kamanyola (900 m) for the cooking cultivars. ‘Mpologoma’, an introduced cultivar, took the shortest time to reach shooting and harvesting at Mushweshwe (406 and 545 days) and Mulungu (410 and 528 days). In contrast, the longest time to maturity was recorded in ‘NARITA 4’ (716 days) at Mushweshwe (Table 7). ‘NARITA 4’ took generally longer to mature at the mid to high altitudes compared with the low altitudes (Table 7).


The local cultivar ‘Barhabeshya’ at 33 kg bunch weight had the heaviest bunch. Generally bunch weight, number of hands and fingers per bunch, and finger length were higher at the low altitude sites (Table 7) and declined with increasing altitude. The bunch weights of ‘Barhabeshya’ (R2= 0.99) and ‘Mbwazirume’ (R2= 0.74) were most strongly correlated with altitude while ‘Matooke’ (R²= 0.09) and ‘NARITA 4’ (R²= 0.15) showed no linear association (Table 6).
The heaviest bunches averaging 39 kg were recorded in ‘NARITA 4’ at Mavivi. Similarly ‘NARITA 4’ bore the highest mean number of hands per bunch (12.3) and fruits per bunch (234), and had longer fruits (22.6 cm) at this site (Table 7). High bunch weights were also obtained from ‘NARITA 2’ at both Mutwanga (37.3 kg) and Mavivi (35.8 kg).
At Kamanyola (900 m), the lowest altitude site, ‘Barhabeshya’ equally had a good bunch weight of 37.2 kg and number of hands (10.3) per bunch. ‘Matooke’ and ‘NARITA 2’ had the poorest performance at Maboya, yielding mean bunch weights of 17.3 and 17.0 kg, respectively (Table 7).
Performance of local and hybrid dessert banana cultivars
‘Gros Michel’ (374 cm), followed by ‘Giant Cavendish’ (349 cm) were the tallest, with the shortest plants (193 cm) observed in the ‘Dwarf Cavendish’ cultivar (Table 6). Plant height declined with increasing altitude across all cultivars. ‘FHIA03’ had the largest mean girth (90 cm) at soil level compared with other dessert banana types (Table 6). Girth declined with altitude only in FHIA03.The number of functionalleaves at flowering varied between 11.8 leaves in ‘Gros Michel’and 8.5 in ‘Giant Cavendish’. There was no significant correlation between the number of functional leaves and altitude in ‘Giant Cavendish’, ‘Dwarf Cavendish’, ‘FHIA03’ or ‘Gros Michel’.
Crop cycle duration generally increased with altitude across the cultivars (R2 = 0.38 to 0.99). For example, the shortest times to flowering of 390, 410 and 435 days were recorded for ‘Gros Michel’, ‘Giant Cavendish’ and ‘Dwarf Cavendish’, respectively at the lowest altitude site of Kamanyola (900 m). ‘FHIA03’ was the best performing cultivar in terms of time to flowering and maturity at the higher altitude sites (Table 6). ‘FHIA03’ (30 kg) and ‘Gros Michel’ (29 kg), respectively had the heaviest bunch weights, with the lightest in ‘Giant Cavendish’. The heaviest bunches were recorded at the low altitude sites of Mavivi, Kamanyola and
Mutwanga. Except in ‘Gros Michel’, where bunch weight significantly (R2=0.88) increased with altitude, bunch weight in the other three cultivars showed no significant correlation with altitude (Tables 8 and 9).
Performance characteristics of beer cultivars across sites
‘Ndundu’ was generally the most robust cultivar in the vegetative stage. It had the greatest plant height (341 cm), girth (81 cm) and number of functional leaves at flowering (11), while ‘Yangambi Km5’ and ‘NARITA 27’ were the least vigorous (Table 7). Plant height significantly increased with increasing altitude in ‘Ndundu’ (R2= 0.7) and ‘Nshika’ (R2= 0.9) while not significantly (R2= 0.01 to 0.2) for ‘Yangambi Km5’and ‘NARITA 27’. The pseudostem girth in ‘Nshika’ significantly (R2= 0.64) declined with the altitude whereas the other cultivars were not affected. The number of functional leaves in ‘Yangambi Km5’ was not correlated with altitude.
The banana beer cultivar ‘ Ndundu’ (AAA-EA) took the shortest time to flower (417 days) and to reach harvest (535 days), while ‘Nshika’, with 531 and 653 days to flowering and harvest respectively, took the longest time (Table 7). The time to harvest generally increased with increasing altitude across the cultivars. ‘Nshika’ (R2= 0.98) followed by ‘Yangambi Km 5’ (R2= 0.83) were more sensitive to the changes in altitude while ‘NARITA 27’ (R2= 0.18) was least sensitive. ‘NARITA 27’ yielded the heaviest bunches (30 kg), and greatest number of hands and fingers and the longest finger length. In contrast, the lightest bunch weight was recorded in ‘Yangambi Km5’ (21 kg). It was noticed that bunch weight significantly increased with increasing altitude in ‘Ndundu’ (R2= 0.89), while it declined for ‘Nshika’ (R2= 0.56). Yields in other cultivars, ‘Yangambi Km5’ (R2= 0.12) and ‘NHS42’ (R2= 0.01) (Table 8) were not significantly associated with altitude.
The different banana cultivars (exotic and local, Table 3) generally responded differently across the three South Kivu sites and four North Kivu sites in eastern DR Congo.
The PC results suggest that both soil factors, especially the level of soil pH, OM, N, P, K, Ca and Mg in the soil and altitude influenced bunch attributes and thus yield of the cultivars. Yield generally increased with increase in OM, N, P, K, Ca and pH. High OM, N, P, K and Ca have been reported to be vital for the growth of the banana plant. A high Ca and OM is reported to improve the availability of P that is easily fixed in the soil under low pH conditions (Pessarakli, 1999). It is thus not surprising that increasing pH was also observed to improve bunch yields. In addition to making P available to the banana plant, higher pH also improves the growth of banana roots, thus improving nutrient and water uptake by plants (Pessarakli, 1999). Altitude was observed to have a negative association with bunch weight, particularly when N, K, P and OM concentrations are low. This could be attributed to the fact that most assimilates go towards sucker development at the high altitudes (Sikyolo et al., 2013; Turner et al., 2016).
The relationship between altitude and time from planting to flowering had a non-linear relationship, with the time from planting to flowering observed to decline at high altitudes. It is understood that PCA uses linear regressions and the curvilinear response may appear as misleading. The planting to bunch emergence data are the means for three crop cycles and will be influenced by the effect of altitude on the time when the ratoon crop began to grow. High altitudes cause bananas to produce suckers at an earlier stage of development compared with plants grown at lower elevations (Sikyolo et al., 2013; Turner et al., 2016). Thus the time from planting to bunch emergence (flowering) when averaged over a number of crop cycles is likely to be different from the data obtained for that cultivar and location for the plant crop. The plant crop data may more accurately show a relationship between altitude and time from planting to bunch emergence than data combined for three crop cycles. For example, time from planting to flowering has been shown to increase with altitude in the plant crop (Sikyolo et al., 2013). This is further strengthened by the fact that the fruit filling phase increases linearly with altitude, that is, is largely free of the effect of early or late suckering that is likely to influence the data on planting to bunch emergence.The performance of the plantain cultivars was influenced by both the altitude and the prevailing soil conditions. Plantains have been reported to grow best at lower elevations (<1200 masl) (Sebasigari, 1985). However, a few plantain cultivars are also found at higher elevations in eastern DR Congo. Vitousek et al. (1994) reported increased rates of soil mineralization along a decreasing elevation gradient which was most strongly associated with altitude, influencing banana production. Despite the ability of plantains to grow in a wide range of soils, optimum nitrogen, potassium and phosphorus are needed to satisfy plant requirements for profitable production (Zake et al., 2000). The introduced plantain cultivars ‘T6’ and ‘Obubit’ had good yields comparable to ‘Musheba’, the local check, at the low altitude sites in this study. These cultivars in addition to having heavier bunches, had shorter production cycles. A shorter crop cycle gives a higher annual yield (Gaidashova et al., 2008) and this is one of the traits desired by farmers. In contrast, ‘FHIA21’ was better adapted to the mid and high altitude sites, outperforming the local check ‘Musheba’ and the other introduced plantain cultivars. Plantain cultivars ‘Obubit’ and ‘T6’ can therefore be promoted with a good level of acceptance in the low altitude areas whereas ‘FHIA21’ can be promoted at the high altitude sites among the communities in eastern DR Congo. ‘FHIA 21’, ‘Obubit’ and ‘Musheba’ also yielded acceptable bunch sizes of above 25 kg at Butembo (1815 masl); and 28 kg at Mutwanga (1049 masl) (Tables 1 and 4). These mean bunch weight are slightly higher than plantain yields of 24 kg per bunch previously reported in Mutwanga (Ndungo Vigheri, personal communication, 2011). Butembo and Mutwanga have a favourable microclimate with excellent volcanic-derived soils with high potassium concentration (Table 2). Optimum potassium is especially vital for vigorous growth of banana as it increases uptake of other essential elements such as nitrogen and phosphorus (Twyford and Walmsley, 1973; Bolanos et al., 2003).
All the green cooking cultivars examined were outperformed by ‘Barhabeshya’ the local check. Other cooking cultivars with relatively good yield were the local cultivars ‘Mukingiro’ and ‘Matooke’ and the introduced cultivar ‘NARITA 2’. Yield of the cooking cultivars was influenced by the interaction between the soils and altitude. For example, a high correlation was observed between altitude and bunch weight in ‘Barhabesha’ and ‘Mbwazirume’ (Table 8). ‘NARITA 4’ only performed well at the low altitude site of Mavivi that has good soil physical characteristics and abundant and well distributed rainfall.
The performance of the dessert cultivars was influenced by changes in altitude, soil conditions and genotype. For example, the number of functional leaves increased with increasing altitude in the ‘Cavendish’ types, while it declined in ‘Gros Michel’ and ‘FHIA03’. These cultivars generally performed poorly at Maboya, with characteristic poor soils. This is consistent with Bolanos et al. (2003) who observed that sufficient soil nutrients are essential in obtaining higher total fruit weight and consequently heavy bunches. The introduced hybrid ‘FHIA03’ significantly outperformed the other cultivars in bunch weight. Dela Cruz et al. (2008) described ‘FHIA03’ as a cultivar that can be grown in diverse soil types, tolerant to prolonged drought, and grows well between 0 and 1,500 m. The intermediate height of ‘FHIA03’ plants and robust pseudostem are desirable traits in the hilly areas where plants are prone to toppling or pseudostem breakage due to wind. Bunch weights in ‘Gros Michel’ increased with altitude, suggesting that it could establish well at these high altitudes with reduced pest and disease problems.
The beer cultivar ‘Ndundu’ was more vigorous, despite the introduced cultivar ‘NARITA 27’ having the best bunch yields and an intermediate crop cycle length. ‘NARITA 27’ also had several hands and long fingers, in addition to not being significantly impacted by the changes in altitude. These attributes make the cultivar ideal for all the evaluated altitudes. ‘Nshika’ was the best performing local cultivar for the low altitude sites. It had yields matching that of ‘NARITA 27’ at the low altitude sites. ‘Nshika’ has been reported as a productive banana cultivar (Dowiya et al., 2009) occupying over 68% of the landscape (Ocimati et al., 2013) across South Kivu. However, the yield of ‘Nshika’ was observed to decline with increasing altitude, suggesting it is better adapted to the low altitude sites. ‘Ndundu’ was more adapted to the high altitude sites (with yields matching that of ‘NARITA 27’ and ‘Nshika’ at low altitudes). This beer cultivar could thus be selectively promoted at the high altitude sites. Furthermore, ‘Nshika’ and ‘Ndundu’ benefit from their shorter production cycles.
CONCLUSIONS AND RECOMMENDATIONS
The results of this study indicate that the tested cultivars are all excellent in terms of yield, even though altitude and soils differently influenced their performance. For instance, at the Mavivi site, all plantains yielded bunch weights ranging from 33.2 kg (‘Obubit’) to 36.6 kg (‘Musheba’). Beer cultivar ‘Nshika’ is more adapted to low altitudes while ‘Ndundu’ is adapted to the high altitude sites. In Mutwanga, all dessert cultivars yielded heavy bunches averaging between 27.4 kg (‘Dwarf Cavendish’) to 41.9 kg (‘FHIA03’). ‘Musheba’ (plantain), ‘Barhabesha’ (green cooking), ‘FHIA03’ (dessert) and ‘NARITA 27’ (beer) were not significantly impacted by altitude. These variations in cultivar adaptability can be exploited by selectively promoting cultivars in specific agro-ecologies/ niches (as influenced by altitude and soils) to which they are more adapted.
Cultivar attributes such as height, pseudostem girth and leaf production can also be exploited. For example, ‘FHIA21’ (plantain), ‘Matooke’ (cooking), ‘Dwarf Cavendish’ (dessert) and ‘NARITA 27’ (beer) have short heights, a desirable trait for easy harvesting and areas prone to strong winds. ‘Musheba’ (plantain), ‘Mpologoma’ (cooking), ‘FHIA03’ (dessert) and ‘Ndundu’ (beer) have the shortest cropping cycles - an important criteria for farmers in selecting cultivars to grow. Furthermore, in comparison with other cultivars, it was noticed that plantains yielded relatively heavy bunches averaging 17.4 kg to 23.4 kg in Maboya, despite this being the site with the lowest soil fertility.
The authors have not declared any conflict of interests.
REFERENCES
|
Bagamba F, Senyonga JW, Tushemereirwe WK, Gold CS (1999). Performance and profitability of the banana subsector in Uganda farming systems. In: Proceedings of an International Symposium: Bananas and Food Security, Douala, Cameroon, 10-14 November 1998. INIBAP, Montpellier, France pp. 729-740.
|
|
|
|
Bolanos M, Morales BHO, Celisal D (2003). Fertilizer (organic and inorganic) and production of 'Dominico Harton'. Infomusa 12:38-41.
|
|
|
|
|
Castillo LE (2002). FHIA-21 hybrid - Characteristics and cultivation recommendations. FHIA.
|
|
|
|
|
CIALCA (2008). Final report Phase I. January 2006 - December 2008. Progress Report 5:8-9.
|
|
|
|
|
Coyne DL, Talwana LAH, Maslen NR (2003). Plant parasitic nematodes associated with root and tuber crops in Uganda. Afr. J. Crop Prot. 9:87-98.
|
|
|
|
|
Daniells JW (2000). Which banana variety should I grow? Informusa (FRA) 9(1):31-33.
|
|
|
|
|
Dela Cruz FS, Gueco LS, Damasco OP, Huelgas VC, dela Cueva FM, Dizon TO, Sison MLJ, Banasihan IG, Sinohin VO, Molina AB (2008). Farmers' Handbook on Introduced and Local Banana Cultivars in the Philippines. Bioversity International, ISBN 978-971-91751-8-6. P 67.
|
|
|
|
|
Dheda DB, Nzawele BD, Roux N, Ngezahayo F, Vigheri N, De Langhe E, Karamura D, Picq C, Mobambo P, Swennen R, Blomme G (2011). Musa collection and characterization in central and eastern DR Congo: a chronological overview. Acta Hortic. 897:87-94.
Crossref
|
|
|
|
|
Dowiya NB, Rweyemamu CL, Maerere AP (2009). Banana (Musa spp. Colla) cropping systems, production constraints and cultivar preferences in eastern Democratic Republic of Congo. J. Anim. Plant Sci. 4:341-356.
|
|
|
|
|
FAO (1985). Report of the workshop on production and marketing constraints on roots, tubers and plantains in Africa, Zaire1, FAO, Rome.
|
|
|
|
|
FAOSTAT (2015). http://faostat3.ao.org/browse/Q/*/E. Accessed on 12th May 2015.
|
|
|
|
|
Gaidashova SV, Karamura F, Karamura EB (2008). Agronomic performance of introduced banana varieties in lowlands of Rwanda. African Crop Science Society. Afr. J. Crop Sci. 16:9-16.
|
|
|
|
|
GenStat (2008). Introduction to GenStat for Windows. 11th Edition. Lawes Agricultural Trust, Rothamsted Experimental Station, UK.
|
|
|
|
|
Gold CS, Karamura EB, Kiggundu A, Bagamba F, Abera AMK (1999). Geographic shifts in highland cooking banana (Musa spp., group AAA-EA) production in Uganda. Int. J. Sust. Agric. World Ecol. 6:45-56.
Crossref
|
|
|
|
|
Gold CS, Pena JE, Karamura EB (2001). Biology and integrated pest management for the banana weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Integrated Pest Manage. Rev. 6:79-155.
Crossref
|
|
|
|
|
Gold CS, Speijer PR, Karamura EB, Rukazambuga ND (1994). Assessment of banana weevils in East African highland banana systems and strategies for control. In: Valmayor, R.V. et al (eds), Proc. Banana Nematode /Borer Weevil Conference. Kuala Lumpur, 18-22 April 1994. INIBAP, Los Banos, Philippines pp. 170-190.
|
|
|
|
|
Gowen SR (1995). Pests. In: Bananas and Plantains (Gowen S.R., ed.). Chapman and Hall, London pp. 382-402.
Crossref
|
|
|
|
|
INIBAP (1996). Annual Report. INIBAP, Montpellier, France.
|
|
|
|
|
INIBAP (2003). Conservation through utilization of bananas and plantains in the Great Lakes region of East Africa - Final Report. INIBAP, Montpellier, France.
|
|
|
|
|
Kamira M, Hauser S, Van Asten P, Coyne D, Talwana HL (2013). Plant parasitic nematodes associated with banana and plantain in eastern and western Democratic Republic of Congo. Nematropica 43:216-225.
|
|
|
|
|
Kamira M, Sivirihauma C, Ntamwira J, Ocimati W, Katungu MG, Bigabwa JB, Vutseme L, Blomme G (2015). Household uses of the banana plant in eastern Democratic Republic of Congo. J. Appl. Biosci. 94:8915-8929.
|
|
|
|
|
Karamura EB (1993). The strategic importance of bananas/ plantains in Uganda. In: Gold, C.S. and Gemmill, B. (eds). Biological and Integrated Control of Highland Banana Pests and Diseases in Africa. Proceedings of a research coordination meeting held in Cotonou, Benin, 12-14 November 1991. pp. 384- 387.
|
|
|
|
|
Krauss U, Figueroa R, Johanson A, Arévalo E, Anguiz R, Cabezas O, García L (1999). Musa clones in Peru: classification, uses, production potential and constraints. InfoMusa 8(2):19-26.
|
|
|
|
|
Mobambo KN, Gauhl F, Swennen R, Pasberg-Gauhl C (1996). Assessment of the cropping cycle effects of black leaf streak severity and yield decline of plantain and plantain hybrids. Int. J. Pest Manage. 42:1-8.
Crossref
|
|
|
|
|
Molina AB, Viljoen A, Fabregar E, Karamura D, Van den Bergh I, Ramillete EB, Sinohin VO, Sheng O, Yi Ganjun (2010). Resistance to Fusarium oxyporum f.sp. cubense in African Bananas. Paper presented at APS, USA.
|
|
|
|
|
Ndungo V, Eden-Green S, Blomme G, Crozier J, Smith J (2006). Presence of banana Xanthomonas wilt (Xanthomonas campestris pv. musacearum) in the Democratic Republic of Congo (DRC). New Disease Reports. Plant Pathol. 55(2):294.
Crossref
|
|
|
|
|
Ndungo V (1997). Agronomic evaluation and Fusarium wilt resistance of the hybrids FHIA-01 and FHIA-03 in Burundi. Infomusa (FRA) 6(1):26-27.
|
|
|
|
|
Nguthi F, Onyango M, Muniu F, Muthamia J, Njuguna M, (1999). Biotechnology to Benefit Small-Scale Banana Producers in Kenya. Annual report, KARI. Nairobi, Kenya.
|
|
|
|
|
Nyabyenda P (2006). Les Plantes Cultivées en Régions Tropicales d'Altitude d'Afrique. Les presses agronomiques de Gembloux, Gembloux, Belgium. pp. 22-24.
|
|
|
|
|
Ocan D, Mukasa HH, Rubaihayo PR, Tinzaara W, Blomme G (2008). Effects of banana weevil damage on plant growth and yield of East African Musa genotypes. J. Appl. Biosci. 9(2):407-415.
|
|
|
|
|
Ocimati W, Karamura D, Rutikanga A, Sivirihauma C, Ndungo V, Adheka J, Dhed'a B, Muhindo H, Ntamwira J, Hakizimana S, Ngezahayo F, Ragama P, Lepoint P, Kanyaruguru JP, De Langhe, Gaidashova SV, Nsabimana A, Murekezi C, Blomme G (2013). Musa germplasm diversity status across a wide range of agro-ecological zones in Rwanda, Burundi and eastern Democratic Republic of Congo. In: Blomme, G. et al. (eds). Banana Systems in the Humid Highlands of Sub-Saharan Africa: Enhancing Resilience and Productivity. CAB International, Wallingford, UK. pp. 8-21.
|
|
|
|
|
Pessarakli M (1999). Handbook of plant and crop stresses. 2nd Edn, Revised and Expanded. Marcel Dekker Inc. New York, USA.
Crossref
|
|
|
|
|
Ploetz R (2004). Diseases and pests: a review of their importance and management. Infomusa 13:11-16.
|
|
|
|
|
Pollefeys P, Sharrock S, Arnaud E (2004). Preliminary Analysis of the Literature on the Distribution of Wild Musa Species Using MGIS and DIVA-GIS. Int. Plant Genet. Resour. Institute, Rome, Italy. P 31.
|
|
|
|
|
SAS Institute (2008). SAS Institute Inc., SAS/STAT User's Guide Version 6, Edition 4, Cary, NC.
|
|
|
|
|
Sebasigari K (1985). Overview of banana cultivation and constraints in the Economic Community of the Great Lakes States (CEPGL). In: Kirby, R. and Ngendahayo, D. (eds), Banana Production and Research In Eastern and Central Africa. Proceedings of a Regional Workshop held in Bujumbura, Burundi 14-17 December 1983. IRAZ/ IDRC. IDRC-MR114e. pp. 9-22.
|
|
|
|
|
Sikyolo I, Sivirihauma C, Ndungo V, De Langhe E, Ocimati W, Blomme G (2013). Growth and yield of plantain cultivars at four sites of differing altitudes in North Kivu, Eastern Democratic Republic of Congo. In: Blomme, G., Vanlauwe, B., van Asten, P. (eds) Banana Systems in the Humid Highlands of Sub-Saharan Africa: Enhancing Resilience and Productivity. CAB International, Wallingford, UK. pp. 48-57.
Crossref
|
|
|
|
|
Silva SO, Alves EJ, Lima MB, Silveira JRS (2002). Bananeira. In: Bruckner, C.H (ed). Melhoramento de Fruteiras Tropicais. Viçosa: UFV. pp. 101-157.
|
|
|
|
|
Simmonds NW (1966). Bananas. 2nd edition. Longman, London, UK.
|
|
|
|
|
Simmonds NW (1995). Banana. In: Smart, J. and Simmonds, N.W. (eds.) Evolution of Crop Plants. Longman, London, UK. pp. 370-375.
|
|
|
|
|
Smales LR (2006). A new acuariid species (Spirurida, Acuariidae) and other nematodes from Hydromys (Muridae, Hydromyinae) from Papua, Indonesia and Papua New Guinea. Zootaxa 1110:27-37.
|
|
|
|
|
Speijer PR (1996). Nematode damage of roots and rhizomes of four Musa cultivars. MusAfrica 9:8-9.
|
|
|
|
|
Speijer PR, Kajumba C, Ssango F (1999). East African highland banana production as influenced by nematodes and crop management in Uganda. Int. J. Pest Manage. 45:41-59.
Crossref
|
|
|
|
|
Turner DW, Fortescue JA, Thomas DS (2007). Environmental physiology of the bananas (Musa spp.). Braz. J. Plant Physiol. 19:463-484.
Crossref
|
|
|
|
|
Turner DW, Fortescue JA, Ocimati W, Blomme G (2016). Plantain cultivars (Musa spp. AAB) grown at different altitudes demonstrate cool temperature and photoperiod responses relevant to genetic improvement. Field Crops Res. 194:103-111.
Crossref
|
|
|
|
|
Tushemereirwe W, Batte M, Nyine M, Tumuhimbise R, Barekye A, Tendo S, Kubiriba J, Lorenzen J, Swennen R (2015). Performance of NARITA banana hybrids in the preliminary yield trial for three cycles in Uganda.
|
|
|
|
|
Tushemereirwe W, Kangire A, Ssekiwoko F, Offord LC, Crozier J, Boa E, Rutherford M, Smith JJ (2004). First report of Xanthomonas campestris pv. musacearum on banana in Uganda. Plant Pathol. 53:802.
Crossref
|
|
|
|
|
Twyford IT, Walmsley D (1973). Mineral composition of the 'Robusta' banana plant. Plant Soil 39:227-243.
Crossref
|
|
|
|
|
van Asten PJA, Wairegi LWI, Mukasa D, Uringi NO (2011). Agronomic and economic benefits of coffee–banana intercropping in Uganda's smallholder farming systems. Agric. Syst. 104(4):326-334.
Crossref
|
|
|
|
|
Vezina A, Van den Bergh I (2016). NARITA hybrids. PROMUSA.
|
|
|
|
|
Vezina A (2016). East African highland banana subgroup. PROMUSA.
|
|
|
|
|
Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994). Litter decomposition on the Mauna Loa environmental matrix, Hawaii: patterns, mechanisms, and models. Ecology 75:418-429.
Crossref
|
|
|
|
|
Yirgou D, Bradbury JF (1974). A note on wilt of banana caused by the enset wilt organism Xanthomonas campestris pv. musacearum. East Afr. J. Agric. For. 40(1):111-114.
|
|
|
|
|
Zake YK, Bwamiki DP, Nkwiine C (2000). Soil management requirements for banana production on the heavy soils around Lake Victoria in Uganda. In: Craenen, K., Ortiz, R., Karamura, E.B. and Vulsteke, D.R. (eds.) Proceedings of the First International Symposium on Banana and Plantain for Africa. Acta Hortic. 540:285-292.
Crossref
|
|