ABSTRACT
The standard germination tests have been commonly used on commercial grain crops, such as soybean, field beans, rice and maize. However, there are no standard tests for potential new crops; quinoa being one of them. This work is aimed at evaluating the effect of substrate, temperature and counting time in seed germination of quinoa. The following treatments were used in tests with seeds: on blotter paper, between blotter paper, on sand and within sand, all previously soaked with distilled water. These substrates were combined to the temperatures of 15, 20, 25, 30°C, and alternated between 20 to 30°C. The experiment was conducted on an entirely randomized factorial design 5 × 4 (temperature × substrate) with 4 repetitions. Normal seedlings, abnormal seedlings and dead seeds were counted until stabilized. The germination velocity index and mean time for germination were calculated. From the results, it was concluded that germination test of quinoa seeds should be conducted at 20 to 30°C alternate temperatures, on or between blotter paper, with initial count at 2 days and final count at 4 days.
Key words: Chenopodium quinoa willd., substrate temperature, counting time, seed viability.
Quinoa (Chenopodium quinoa Willd.) is a plant of the Amaranthaceae family. The genus Chenopodium comprises of 150 species which are encountered in America, Asia and Europe (Bazile and Baudron, 2014). It is a dicotyledonous plant with ample variability in germplasm, including halophytic ecotypes, soil salinity tolerance, and xerophytes; thriving and reproducing under low moisture conditions (Jellen, 2014). The species originated in around the Titicaca Lake in the Andean mountain range of Bolivia and Peru. This pseudocereal has been largely consumed by local indigenous populations of Bolivia, Peru, Ecuador, Chile, Argentina and Colombia. For centuries, it was one of the main components of daily diet for these peoples. Its cultivation declined during the colonial era, when the conquerors introduced wheat and barley crops. However, in recent times, its popularity has grown in Europe, North America and the Andean region; mainly associated with the needs of those adopting vegetarian diet and those with bias towards gluten and lactose (Jellen, 2014). From all food grains, quinoa is the only available grain with natural amino acid balance in its protein. Its high quality is expressed by the content of histidine, isoleucine, leucine, phenylalanine, threonine, tryptophan, valine and, mostly, lysine and methionine, the essential amino acids (Stikic et al., 2012). Its grains are rich in minerals (K, Ca, P, Mn, Zn, Cu, Fe and Na), dietetic fibers and vitamins C and E (Dini et al., 2010). These nutritional qualities have turned quinoa into a reference crop, adaptable to various worldwide growing conditions becoming an option to increase food security (FAO, 2011).
The research has intensified in Brazil with the aim of inserting quinoa crop into production systems. However, it has intrinsic problems relating to seed quality, leading to failure in the field (Sigstad and Garcia 2001; Sigstad and Prado 1999). Seed is the most important component in crop establishment, with direct impact on crop performance and productivity (Azevedo, 2003), and the seed research on quinoa is rather incipient. In Brazil, where the crop has a short history, the first recommended cultivars have shown the seed quality problems and there is no specific standard germination test (Brasil, 2009). Germination test, deterministic of the sowing rate, analyses seeds of different batches and allows comparison. Germination depends on the intrinsic characteristics of seeds and environmental factors, such as temperature, moisture and substrate. The latter can be manipulated and adjusted to optimize germination rate, velocity and uniformity. However, the quinoa seeds have a peculiar structure in regard to the cereals, especially the unique pericarp, an outer layer of the dead cells surrounding it, which is a fruit of achene type (Burrieza et al., 2014). Temperature affects the velocity of imbibition and the chemical reactions occurring in the germination process that influences the uniformity and overall germination rate (Carvalho and Nakagawa, 2012). Seeds of different species have shown unique reaction to temperature, which is optimal when they express the maximum potential of germination in the least period (Borges and Rena, 1993; Popinigis, 1985). Some species respond to alternating temperatures, while others best germinate under constant temperature (Alves et al., 2011). The substrate (physical support in which the seed is placed) functions at maintaining ideal conditions for germination and emergence. The structure, aeration, water retention capacity and presence of plant pathogenic organisms can influence seed performance (Figliolia et al., 1993). The choice of substrate should take into account the seed characteristics, such as size, necessity of water and light, counting facility and seedling evaluation (Popinigis, 1985). The quinoa seed structure and its physiology can also influence storage and germination at higher temperatures than what’s prevailing in the high mountains of its origin (Souza et al., 2016). Given the peculiarities of seed plant species that could affect germination, this work aimed at developing methodology to evaluate germination and to establish a standard for routine in quinoa seed quality assessment.
The work was conducted in the Seed Technology Laboratory of the Faculty of Agronomy and Veterinary Medicine (FAV), University of Brasília, Brazil. The quinoa seeds were of cv. BRS Syetetuba, harvested from a field plot grown in Água Limpa Farm, UnB, between March and August, 2015. The plots were hand harvested when seeds were at physiological maturity and contained 20 to 30% water. After harvest the plants were dried at low air moisture condition; during the dry season, threshed, seeds were cleaned and stored in cold room at 10°C temperature at 12% water content. Before initiating the tests, seeds were immersed in 2% sodium hypochlorite for 10 minutes and rinsed with distilled water to avoid fungal infection. The treatments combined of 5 constant temperatures of 15, 20, 25, 30°C, and alternated 20 to 30°C with 4 substrates: on blotter paper (OP), between blotter paper (BP), on sand (OS) and within sand (WS). For the BP, seeds were distributed over two paper sheets previously soaked using 2.5 times the mass of dry paper, covered with a third sheet of paper and placed in a transparent plastic boxes of 11 × 11 × 3 cm. For the OP, the seeds were placed on transparent plastic boxes, containing two sheets previously soaked in distilled water at the same rate as in BP. The sand germination was evaluated in transparent plastic boxes, keeping the same distance between seeds as in the paper test. Seeds were placed on top of sand (OS) and at 1 cm depth (WS). In both treatments, the sand was watered and kept at 60% field capacity (Brazil, 2009).
The effects of temperatures and substrates on seed germination were evaluated by daily count. Seedlings that had radicle longer than 2 cm were scored and the duration of test was determined by the number of days until germination was stabilized and results were expressed in seedlings percentage. Concomitant to germination test, germination velocity index (GVI) and germination mean time (GMT) were calculated. The normal seedling count was conducted daily, while the indexes were obtained by Maguire (1962) and Labouriau (1983), as follows:
GVI = G1/N1+G2/N2+ ... +Gn/Nn
Where, GVI = germination velocity index; G1, G2, Gn = number of normal seedlings at first count (G1), second count (G2) and last count (Gn);N1, N2, Nn = number of days to first count (N1), second count (N2) and last count (Nn);GMT: , em que; GMT: germination mean time (days); ni: number of germinated seeds at each count interval, and ti: time between first and ith count.
The experimental design was an entirely randomized factorial, combining 5 temperatures and 4 substrates (5 × 4), with 4 repetitions. The germination test percent data were transformed by arc sen , to fit normal distribution and the original means was compared by Tukey test at p=0.05. For the statistical analysis the SISVAR 5.3 Software was used (Ferreira, 2011).
There was significant interaction (p<0.05) for all the factors, including temperature × substrate interaction.
The germination rate (%) for between blotter paper (BP) and on blotter paper (OP) was higher for 20 to 30°C alternated temperature (Table 1). However, these results did not differ statistically from those of 20, 25 and 30°C, for BP, and of 15 and 30°C for OP treatment. The within sand seeds had the best germination rate, at 15 and 25°C and, when seeds were placed on sand, there were no statistical differences for temperatures. With the exception of 15 and 25°C, which showed no statistical differences for substrates, the other three temperature treatments had the highest values when the seeds were tested between papers. At 30°C, there was no significant difference and alternated temperature for within sand and on paper tests. Similar results for tests within paper were obtained for malabar spinach (Basella alba, Basellaceae) seeds (Lopes et al., 2005).The alternated 20 to 30°C temperature had the best germination, for both blotter paper tests. These results could be explained by the association of fluctuating in temperatures occurring in the environment of origin of plant species (Borges and Rena, 1993). Quinoa originated in the Andean mountains, where low night temperatures alternate with high day temperatures in low moisture environment. The variations in temperature could cause activation of enzymes relating to germination (Vázquez-Yanez, 1984). Moreover, for the higher germination could be the effect of variable temperature on the seed tegument. The seeds then become more permeable to water and oxygen, influencing the balance between promoting and inhibiting substances (Alves et al., 2015).
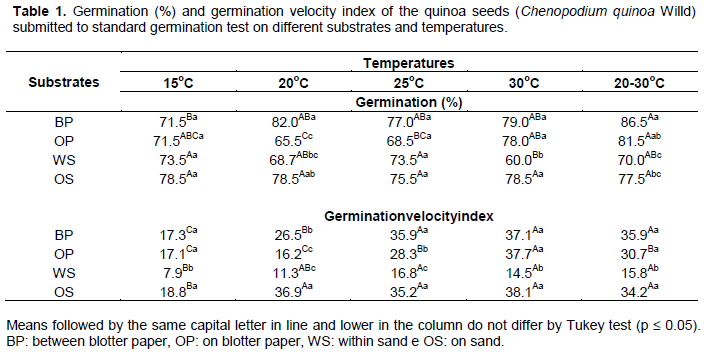
There were no significant differences in germination velocity index (Table 1) for 25, 30 and 20 to 30°C alternated, for seeds placed between paper, within sand and on sand. For seeds placed on blotter paper, the highest germination value was at 30°C, followed by 25°C and 20 to 30°C alternated temperatures. These results show a visible influence of high temperature on germination velocity of quinoa seeds, varying between 15°C low and 30°C high. Low temperatures could reduce enzymatic activities involving seed metabolism, reducing rate and delaying germination (Caldeira et al., 2015). The germination velocity index (GVI) and the germination mean time, at all temperatures, within sand substrate had the lowest values (Table 1 and Figure 1). There was a 50% higher reduction in the GVI and a longer period of 5 days was necessary for germination in comparison with other substrates. These results could be explained by the amount of water in sand blocking oxygen around seeds, reducing respiration and delaying or paralyzing germination (Carvalho and Nakagawa, 2012; Borges and Rena, 1993). Therefore, placing the seeds in sand was inefficient to evaluate germination and vigor of quinoa. Similar results were obtained with Cucumis metuliferus seeds (Alves et al., 2014). Irrespective of substrate, quinoa seeds germinated in less time when they were exposed to 25, 30 and 20 to 30°C, differing from 15 and 20°C temperatures (Figure 1). For within paper substrate at 15°C 2.1 days was necessary, while at 25, 30 and 20 to 30°C, the quinoa seeds germinated in 1.5 days. When seeds were placed on sand and on paper at 30°C, germination occurred on the first day, whereas at 15 e 20°C two days was needed for paper. Seeds placed on sand at 15°C germinated in 2.2 days.
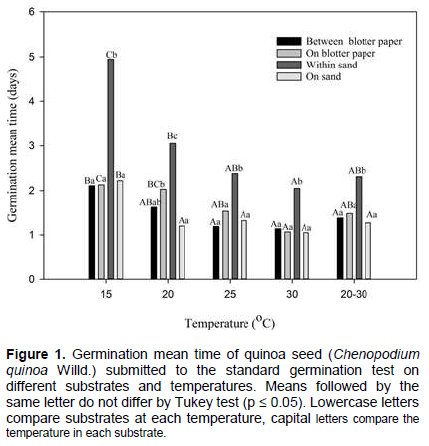
Velocity of germination can be influenced by temperature. When seeds are exposed to low temperature, in general, there was a delay in germination from reduced metabolic activities. On the contrary, at high temperatures seeds germinated more rapidly as a result of protein denaturation coming from increased metabolic activities (Marcos-Filho, 2015). Daily monitoringof quinoa seeds allowed a defined evolution curve for each of the treatments (Figure 2). High germination rates were scored when seeds were placed between paper sheets and on paper sheet for the 20 to 30°C alternated temperature. In general, germination was observed from the first day of the test and on the 2nd day the germination rates were higher than 50%, increasing until stabilized from the 4th day. Therefore, the ideal date for first count should be on the 2nd day and for the second count on the 4th day after sowing. The experiment followed similar criteria to define standard germination test with other crops (Alves et al., 2014, 2015; Caldeira et al., 2015; Oliveira et al., 2014). On soybean and maize, first and second count occurs at 5 to 8 and 4 to 7 days, respectively. On beet root, of the same botanical family as quinoa, first count is done at the 4th day and the second on the 10th day (Brasil, 2009). The more rapid germination on quinoa can be related to the high imbibition capacity of its seeds, causing roots to protrude within 6 to 10 h after being exposed to water (Souza et al., 2016; Makinen et al., 2014). The pericarp consisting of layer of dead cells surrounding the seeds is highly permeable while the tegument and the endotesta are completely consumed during the seed formation, remaining only the exotesta (Burrieza et al., 2014). The external permeability adds up to the water absorption ability of the perisperm formed by large and thin-walled cells, containing highly hydrophilic starch granules (López-Fernandez and Maldonado, 2013). These mechanisms that accelerate seeds germination may have resulted from the environmental conditioners. Around the Titicaca Lake in the high Andes of Bolivia and Peru, the probable center of origin of quinoa, the moisture is reduced. Therefore, the species developed efficient water use during the plant cycle, starting with rapid germination.
The germination test for quinoa seeds using 20 to 30°C, is optimized when seeds are placed in between or on blotter paper, with initial count at 2 days and final count at 4 days from the start of test.
The authors have not declared any conflict of interest.
The authors are thankful to CAPES and the Universidade de Brasília for scholarship and support to the research.
REFERENCES
|
Alves CZ, Candido ACS, Oliveira NC, Lourenço FMS (2014). Teste de germinação em sementes de Cucumis metuliferus E. Mey. Cienc. Rural 44 (2):228-234.
Crossref
|
|
|
|
Alves CZ, Godoy AR, Corrêa LS (2011). Adequação da metodologia para o teste de germinação de sementes de pitaia vermelha. Cienc. Rural 41 (5):779-784.
Crossref
|
|
|
|
|
Alves CZ, Silva JB, Cândido ACS (2015). Metodologia para a condução do teste de germinação em sementes de goiaba. Rev. Ciênc. Agron. 46 (3):615-621.
|
|
|
|
|
Azevedo MRQA, Gouveia JPG, Trovão DMM (2003). Influência das embalagens e condições de armazenamento no vigor de sementes de gergelim. Rev. Bras. Eng. Agríc. Ambient. 7(3):519-524.
Crossref
|
|
|
|
|
Bazile D, Baudron F (2014). Dinámica de expansión mundial del cultivo de La quinua respecto a su alta biodiversidade. In: Bazile D, Bertero D, Nieto C (Eds). State of the art reporto n quinoa around the Word in 2013.Oficina Regional de la FAO para América Latina y el Caribe: Santiago, Chile pp. 49-64.
|
|
|
|
|
Borges EEL, Rena AB (1993). Germinação de sementes. In: Aguiar IB, Pi-a-Rodrigues FCM, Figliolia MB (Eds.). Sementes florestais tropicais. Brasília: ABRATES pp. 83-136.
|
|
|
|
|
Brazil (2009). Ministério da Agricultura Pecuária e Abastecimento. Equipe Técnica de Sementes e Mudas. Regras para análises de sementes. Brasília, DF 398 p.
|
|
|
|
|
Burrieza HP, Lopez-Fernández MP, Maldonado S (2014). Analogous reserve distribution and tissue characteristics in quinoa and grass seeds suggest convergent evolution. Plant Sci. (5):1-11.
Crossref
|
|
|
|
|
Caldeira TL, Beskow S, Mello CR, Faria LC, Souza MR, Guedes HAS (2015). Modelagem probabilística de eventos de precipitação extrema no estado do Rio Grande do Sul. Rev. Bras. Eng. Agríc. Ambient. 19(3):197-20.
Crossref
|
|
|
|
|
Carvalho NM, Nakagawa J (2012). Sementes: ciência, tecnologia e produção. 5.ed. FUNEP: Jaboticabal 590p.
|
|
|
|
|
Dini I, Tenore GC, Dini A (2010). Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. Food Sci. Technol. 43 (3):447-451.
Crossref
|
|
|
|
|
FAO (2011). Oficina Regional para América Latina y el Caribe. La quinua: cultivo milenário para contribuir a la seguridade alimentaria mundial, Bolívia.
|
|
|
|
|
Ferreira DF (2011). Sisvar: A computer statistical analysis system. Ciênc. Agrotecnologia 35(6):1039-1042.
|
|
|
|
|
Figliolia MB, Oliveira EC, Pinã-Rodrigues FCM (1993). Análise de sementes. In: Aguiar IB, Pinã- Rodrigues FCM., Figliolia MB (Eds.). Sementes florestais tropicais. Brasília: ABRATES pp. 137-174.
|
|
|
|
|
Jellen EN, Maughan PJ, Fuentes F, Kolano BA (2014). Botánica, Domesticación y Circulación de Recursos Genétcos In: Bazile D, Bertero D, Nieto C (Eds). State of the art reporto n quinoa around the Word in 2013.Oficina Regional de la FAO para América Latina y el Caribe: Santiago, Chile pp. 11-35.
|
|
|
|
|
Labouriau LG (1983). A germinação das sementes: programa regional de desenvolvimento científico e tecnológico. Washington: Secretaria Geral da Organização dos Estados Americanos 174p.
|
|
|
|
|
Lopes JC, Capucho MT, Martins Filho S, Repossi P (2005). A Influência de temperatura, substrato e luz na germinação de sementes de bertalha. Rev. Bras. Semen. 27(2):18-24.
Crossref
|
|
|
|
|
López-Fernandez MP, Maldonado S (2013). Programmed cell death during quinoa perisperm development. J. Exp. Bot. 57:3747-3753.
Crossref
|
|
|
|
|
Marcos-Filho J (2015). Fisiologia de Sementes de Plantas Cultivadas. Londrina: ABRATES 659 p.
|
|
|
|
|
Maguire JD (1962). Speed of germination aid in selection and evaluation for seeding emergence and vigor. Crop Sci. 2(2): 76-177.
Crossref
|
|
|
|
|
Makinen OE, Hager AS, Arent E (2014). Localization and development of proteolytic activities in quinoa (Chenopodium quinoa) seeds during germination and early seedling growth. J. Cereal Sci. 1(6):1-6.
|
|
|
|
|
Oliveira IVM, Cavalcante IHL, Beckmann MZ, Martins ABG (2005). Temperatura na germinação de sementes de sapota-preta. Rev. Biol. Ciênc. Terra 5(2):1-7.
|
|
|
|
|
Popinigis F (1985). Fisiologia da semente. 2. ed. Brasília: AGIPLAN 289 p.
|
|
|
|
|
Sigstad EE, Garcia CL (2001). A microcalorimetric analysis of quinoa seeds with different initial water content during germination at 25oC. Themochim. Acta 366(2):149-155.
Crossref
|
|
|
|
|
Sigstad EE, Prado FEA (1998). Microcalorimetric study of Chenopodium quinoa Willd. seed germination. Themochim. Acta 326(1-2):159-164.
Crossref
|
|
|
|
|
Stikic R, Glamoclija D, Demin M, Vucelic-Radovic DMO, Jacobsen SE, Milavonic M (2012). Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J. Cereal Sci. 55(2):132-138.
Crossref
|
|
|
|
|
Souza FFJ, Devilla IA, Souza RTG, Spehar CR (2016). Physiological quality of quinoa seeds submitted to different storage conditions. Afr. J. Agric. Res. 11(15):1299-1308.
Crossref
|
|
|
|
|
Vázquez-Yanez C, Orozco-Segovia A (1984). Fisiologia ecológica de lãs semillas de árboles de la selva tropical: um reflejo de su ambiente. Ciência 35:191-201.
|
|