ABSTRACT
Synthetic pesticides used in agriculture to control pathogens are being widely questioned for their harmful effects on human health and the environment. Therefore, the objective of this study was to evaluate the fungistatic activity of Lippia sidoides Cham. essential oil on alternative control of Curvularia lunata (Wakker), the causal agent of spot in maize plants. The fungitoxic contact effect was successful, resulting in a strong inhibition of C. lunata at the concentration of 50 mg mL−1. Complete adverse effect on the germination of the conidia of C. lunata was obtained at concentrations of 5 and 7.5 mg mL−1. In the preventive assay in maize plants, that is, effect of essential oil on C. lunata, a reduction in the progression of the disease was observed at the oil concentration of 7.5 mg mL−1. Regarding the curative effect in maize plants, no satisfactory result was obtained at concentrations from 0.625 to 7.5 mg mL−1. Cytotoxic test in animal cells was also performed by the viability test using MTT assay, which showed that none of the analyzed concentrations was toxic to the cells. These results demonstrate the potential effect of the essential oil of L. sidoides on the prevention and inhibition of mycelia and conidia germination, controlling the phytopathogenic fungus C. lunata.
Key words: Phytopathogenic fungus, effect preventive, effect curative, cytotoxicity.
The production of maize (Zea mays L.) can be affected by a number of factors such as climate, cultural practices, and especially, phytopathogenic microorganisms. All the plant species are potential hosts of fungi that can be primarily associated to different parts of the plant (Agrios, 2005). A majority of them cause negative impact by causing diseases in plants and, therefore, affecting the yield of the harvested products (Bennett, 1998). This necessitates research for the development of new alternatives for the control of pathogenic organisms, where productivity and quality can be high (Okumura et al., 2011). Several filamentous fungi are cultivated and exploited commercially for the production of enzymes and metabolites. Since the past few years, bioactive compounds have become popular and are the target of numerous surveys (Archer et al., 2008; Sun et al., 2010). In addition, several plants, whose primary mechanism of action may be the inhibition of mycelial growth, are being studied for their fungitoxicity in the control of pathogens (Milanesi et al., 2009). According to the literature, Brazil is the third largest consumer of pesticides in the world, besides being the third in mortality rate due to cancer diseases (Krawczyk et al., 2017). Synthetic substances used in agriculture to control pathogens such as benzimidazoles, aromatic hydrocarbons, and inhibitors of the biosynthesis of sterols are being widely questioned for their harmful effects on human health and the environment. These adverse effects such as pollution of air, water, and food and the growth of resistance of microorganisms such as Pyricularia grisea (Sacc.), Aspergillus species, and Colletotrichum musae (Berk. & M.A. Curtis) fungi have led to the development of resistance to the most widely used fungicides (Aguiar et al., 2014).
Recent studies indicate an increase in diseases caused by the fungus Curvularia lunata (Wakker) in maize cultivars in China, and consequently, a major loss in maize production (Gao et al., 2012). The maize leaf disease destroys the photosynthetic tissues and directly affects the formation of grains. Among the maize diseases, the spot of Curvularia occurs less often in places of warm climate, but it is prevalent and causes losses in tropical regions (Reis et al., 2004). The destruction of 25% of foliar area of maize in its terminal portion, near the flowering period, can reduce 32% of the production (Fancelli, 1998). There is a shortage of studies on this disease in Brazil, and there are hardly any reports of Curvularia species. in Brazil. However, the literature reports a marked increase in the incidence and severity of this disease. Currently, several new biobased products with antimicrobial action have been studied, especially essential oils from medicinal plants (Muthaiyan et al., 2012; Vaz-De-Melo et al., 2010). Considering the growing importance of Curvularia spp. in maize, the few studies conducted for the control of this disease in tropical areas, the growing search for alternative methods of control, and the potential antifungal agents from vegetable oils from medicinal plants, the objective of this study was to evaluate the fungistatic activity and cytotoxity effect of Lippia sidoides essential oil on Curvularia spp.
This study was carried out in the Laboratory of Phytopathology of the Federal University of Tocantins, Gurupi Campus, Tocantins, Brazil.
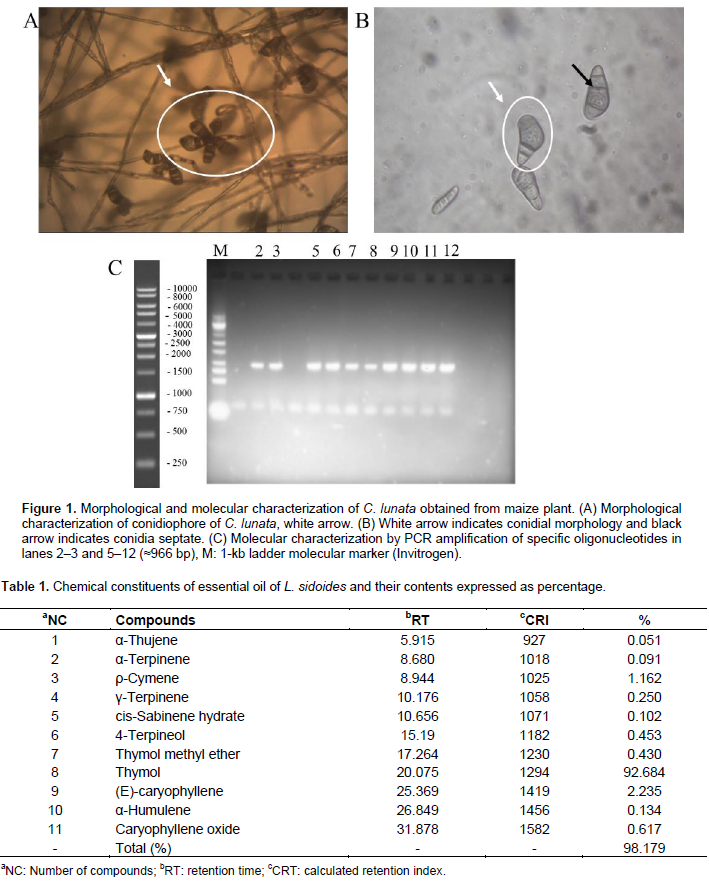
Morphological and molecular characterization of C. lunata
The fungus C. lunata was initially isolated from maize plants from the experimental field of UFT -Gurupi with symptoms of the disease and grown in potato dextrose agar (PDA) culture medium and deposited in a collection library of CENARGEN (Embrapa Genetic Resources and Biotechnology) under the number CEN 1095. The morphological characterization of C. lunata was verified through observations of the macroscopic and microscopic characteristics according to previous studies (Ferreira et al., 2010; Kern and Blevins, 1999). For the molecular characterization done according to Hou et al. (2013), DNA extraction was performed using a cetyl trimetyl ammonium bromide (CTAB) method described by Zolan and Pukilla (1986). The primers used were P1: (5ʹ-ATG GAC GAG AAC AAC AGG ATAA CGA-3ʹ) and P2: (5ʹ-CTA CCA GCA TTT GAA TTT ACT CCAG-3ʹ). The amplification was conducted in a Techne TC-5000 Thermal Cycler (Techne). PCR was carried out using Taq DNA polymerase (Invitrogen) and the PCR program was performed as follows: 4 min at 95°C, 30 cycles at 95°C for 1 min, annealing at 55°C for 2 min and 72°C for 2 min, and a final step at 72°C for 5 min, to obtain the PCR products.
Plant material and steam distillation
L. sidoides originating in Ceará was collected in Gurupi (11°44ʹ48ʺ latitude S, 49°02ʹ55ʺ longitude W), Tocantins, Brazil. Taxonomic identification was confirmed by experts at the herbarium (Federal University of São João Del Rei, Brazil), where samples were deposited with reference number 8303. L. sidoides essential oil was extracted from the leaves by steam distillation method in a Clevenger-modified apparatus, as described by Guimarães et al. (2008) and stored at 4°C until further analysis, before antifungal experiments were conducted.
Gas chromatography-mass spectrometry (GC-MS) analysis
Qualitative analyses were performed through gas chromatography coupled to mass spectrometry (GC-MS) using the Shimadzu GC-2010 model equipped with selective detector for the mass Model QP2010Plus, with the equipment operated under the following conditions: fuzed silica capillary column RTX-5MS (30 m × 0.25 mm × 0.25 μm film thickness), with the following schedule of temperature in the column: 60 to 240°C (3°C/min), temperature of the injector 220°C, helium gas carrier, injection with rate of split (1:100) with injected volume of 1 µL of a solution 1:1000 in hexane. For the mass spectrometer (MS), the following conditions were used: impact energy of 70 V and temperature of the source of ions and the interface at 200°C. A homologous series of n-alkanes (C9H20…C26H54) were injected under the same conditions as for samples. The constituents were identified by comparing their spectra of masses with those from the databases from the Nist and Wiley 229 libraries and also by comparing between their rates of retention calculated using those reported in the literature (Adams, 2007). The quantification of the levels of the compounds, expressed as a percentage based on the standardization of areas, was obtained by using a gaseous chromatograph equipped with a detector flame (DIC), using a diagnostic Shimadzu GC-2010, in the following experimental conditions: a capillary column RTX-5MS (30 m × 0.25 mm × 0.25 μm film thickness); temperature of the injector at 220°C; temperature of the DIC 300°C; programing the column: initial temperature of 60°C with a heating rate of 3°C/min up to 240°C, then increasing to a heating rate of 10°C/min up to 300°C and remaining at this temperature for 10 min; nitrogen drag gas (1.18 mL min−1); rate of split 1:50; pressure in the column of 115 kPa, and injected volume of 1 µL, diluted in hexane (1:100 v/v). The calculated retention index was performed according to Mühlen (2009).
Pathogenicity of C. lunata
The pathogenicity of C. lunata was verified according to the methodology of Sá et al. (2011) by testing the inoculations in maize plants. Maize seeds were sown in pots made of polyethylene containing substrate and 10 g of a commercial fertilizer in each pot. Nine pits were made and two seeds were added to each pit. There was no need to perform pruning. When the plants reached two pairs of true leaves, the inoculation could be initiated using a manual spray (capacity 500 mL) containing the conidial suspension of C. lunata incubated for 10 days in biochemical oxygen demand (BOD) at 25°C at concentrations of 101, 102, 103, 104, 105, and 106 conidia mL−1 until the point of outflow on the leaves. Positive controls were prepared using only sterile distilled water with the same volume used for concentrations of conidia. Then, the pots were maintained for 48 h with humid cotton and sealed with a plastic bag to provide a humid chamber. After 48 h of inoculation, the pots were left in a shaded place until the appearance of the first symptoms of leaf spot of Curvularia.
Phytotoxicity of essential oil of L. sidoides
The experiment for testing the phytotoxicity was carried out under greenhouse conditions (relative humidity of 70 to 80% and temperature at 27 to 33°C) according to the methodology used by Santos et al. (2013). For planting of maize, Traktor (Syngenta®) seeds were used by seeding two seeds in each pit. After sowing, the pots were irrigated daily until the growth of the seedlings reached four definite leaves or till 15 days of planting. Only the manual spray trigger was used for the application of treatments, coupled to a 10-mL test tube containing the solution. Each pot was sprayed with 5 mL of 2.5 to 50 mg mL−1 solutions. After 24 h of application, the evaluation was performed according to the scale of phytotoxicity adapted by Freitas et al. (2009).
Effect of L. sidoides essential oil on mycelia C. lunata
To verify the effect of essential oil on the mycelial growth of the phytopathogen, 100 µL of each of the five solutions (2.5, 5, 7.5, 10, and 50 mg mL−1) was spread on the surface of the culture medium. Then, a disk of mycelium-agar of 6 mm in diameter was placed in the center of the plates and incubated in BOD at 25°C for 10 days according to the methodology of Seixas et al. (2011). The evaluations were carried out by measuring the diameter of the mycelial outlining of two orthogonal axes with each other over the center of the plates, resulting in an arithmetic mean, and measured every 2 days (2, 4, 6, 8, and 10 days). Two controls, one containing Tween 80% (0.03%) in sterile distilled water (positive control) and the other containing methyl thiophanate (fungicide and negative control) at 2 mg mL−1), were tested. All experiments were conducted in triplicate. The regression equations were adjusted for the quantitative factor using a program for preparation of spreadsheets, SigmaPlot 12.0.
Assay of inhibition of conidial germination
This assay was conducted in a completely randomized design with three replications. An aliquot of 50 µl of the conidial suspension of C. lunata (104 conidia mL−1) and another of 50 μL at different concentrations (0, 0.625, 1.25, 2.5, and 5 mg mL−1, respectively) of L. sidoides essential oil containing Tween 80% (0.03%) were placed in each of the containers (“little wells”) of 96-well tissue cultures plates, round bottom (TPP®) (Balbi-Peña et al., 2006). They were incubated in a humid chamber in a photoperiod of 12 h for a total time of 24 h. A total of 200 conidia were counted per treatment by observing the germinated and ungerminated conidia under an optical microscope (Aguiar et al., 2014). The data were analyzed using regression analysis.
Effect of L. sidoides essential oil on C. lunata in maize plants
On each plate containing an inoculum of 7 days of incubation, 10 mL of sterile distilled water was added for the preparation of conidial solutions. The concentration of 104 mL−1 of conidia was chosen for assessing the biological activities. Then, the vessels were maintained for 48 h with a humid and sealed cotton with a plastic bag to provide a humid chamber. After 48 h of inoculation, the pots were left in a shaded place until the appearance of the first leaf symptoms of Curvularia spot (Santos et al., 2013). For evaluating the severity of the disease, a grading scale was used according to Santos et al. (2005), where 0 = healthy plant, 1 = <1% of leaf area sick, 3 = 1 to 5% of the leaf area sick, 5 = 6 to 25% of the leaf area sick, 7 = 26 to 50% of the leaf area sick, and 9 = >50% of the leaf area sick.
Preventive effect of essential oil
To assess the preventive effect of essential oil, a completely randomized experimental design in a factorial design was used with three replicates, where the factors were a type of oil and the following five concentrations of the solutions of oil: 0.625, 1.25, 2.5, 5, and 7.5 mg mL−1. As a positive control, plants sprayed with Tween 80% (0.03%) in sterile distilled water were used, whereas plants sprayed with 2 mg mL−1 methyl thiophanate (fungicide) served as the negative control. For each treatment, 5 mL was sprayed on each pot, and after 1 h, it was inoculated with 5 mL of 104 mL−1 C. lunata conidia. The severity of the disease was assessed every 2 days after the inoculation (five assessments in total) (Santos et al., 2013).
Curative effect of essential oil
To evaluate the curative effect of essential oil, a completely randomized experimental design in a factorial design was used with three replicates, where the factors were a type of oil (the same applied for assessing the preventive effect) and the following five concentrations: 0.625, 1.25, 2.5, 5, and 7.5 mg mL-1. The controls were also the same as described for assessing the preventive effect. The maize plants were inoculated with 5 mL of 104 mL−1 conidial solution, and then the vessels were kept for 48 h with a humid sealed cotton with a plastic bag to provide a moist chamber. After 48 h of inoculation, the pots were left in a shaded place until the appearance of the first leaf symptoms of Curvularia spot. For each treatment, 5 mL was sprayed on each pot after checking the onset of the disease, and the severity of the disease was assessed every 2 days (five assessments in total) after the application of the solutions of oil. Using the results obtained in these assessments, the area under the disease progression curve (AUDPC) was calculated, according to Schneider et al. (1976).
Cytotoxicity of L. sidoides essential oil on (Mesocricetus auratus W.) hamster cells
To verify the cytotoxicity effect of L. sidoides essential oil, mammalian cells (M. auratus), hamster cells (blood, spleen, and liver), were used according to the methodology of Riss et al. (2013). The cells were washed twice in phosphate-buffered saline (PBS), filtrated once (pH 7.2), and centrifuged at 2000 rpm for 10 min/TA. After counting, the cells (1 × 105 cells/mL) were incubated (100 µl) with 50 µl of five concentrations (625, 250, 2500, 5000, and 7500 µg mL−1) of the solution of essential oil of L. sidoides control (water + tween 80 to 0.03%) for 48 h in a 96-well plate. After 48 h, the plate was incubated with 20 µl of [3-(4.5-dimetthythiazol-2-yl)-2.5 diphenyltetiazolium (MTT) bromide] at a concentration of 2.5 mg mL−1 for 4 h at 37°C. Then, the supernatant was aspired from the wells and added to 200 μL of 0.04 M HCl solution in isopropanol or sodium dodecyl sulfate (SDS) to under the crystals of formazan. The plate was read at a wavelength at 540 nm.
Statistical analysis
Results were expressed as mean ± standard error of mean. Linear regression analysis was performed using SISVAR 4.6 according to Ferreira (2001), and graphs were produced using SigmaPlot 12.0. A p-value less than 0.05 was considered statically significant.
The C. lunata isolate was morphologically and molecularly characterized (Figure 1a, b, and c). In PDA culture media, it presented thin aspect of its mycelia; the reverse side of the colony had a dark color with the presence of conidia after 7 days of incubation and forms of regular board and with gray pigmentation and the pattern of amplification by PCR with the expected size (≈966 bp) C. lunata (Figure 1c). Typical symptoms of maize leaf burning were recorded after the incubation period of 48 h after inoculation of plants. Among the six concentrations of conidia prepared (101 to 106 mL−1 conidia), there was a higher incidence of leaf spots with the concentration of 104 mL−1 conidia. However, all the suspensions caused lesions on the leaves of maize plants. The concentration of 104 mL−1 conidia was chosen for assessing the fungal toxicity and the preventive and curative effects in the later stages. According to Agrios (2005), a pathogen can lose its ability of parasitism in relation to its host if successive subcultures in a culture medium were performed. Therefore, in all the isolation procedures, the fungus was picked only once. The chromatographic analysis of essential oil of L. sidoides revealed its chemical constituents, followed by their retention times and the rates of retention calculated and charted, and their levels were expressed as percentage (Table 1).
The primary constituents included thymol (92.684%), (E)-caryophyllene (2.235%), and ρ-cymene (1.162%) (Table 1). The effect of essential oil on the mycelium (mm) of C. lunata when submitted to essential oil of L. sidoides is shown in Table 2 and Figure 2. Fungal growth was observed at concentrations from 2.5 to 10 mg mL−1. As for the concentration 50 mg mL−1, a strong inhibition of C. lunata was observed (Table 2). Comparing the mean values of mycelial growth of the pathogen for 2 and 4 days after incubation, it was observed that the essential oil of L. sidoides promoted the inhibitory effect at the concentration 5 mg mL−1 (Table 2), where it can be seen that in all the concentrations tested, the mean values differ among themselves (Table 2). The fungus exhibited mycelial growth inhibition when submitted to 2.5 and 5 mg mL−1 for 2 days and only at 7.5 mg mL−1 for 2 and 4 days. The concentration 2.5 mg mL−1 was not effective in reducing the mycelial growth over time (Table 2). This comparison may be more evident if the growth of positive control was examined in relation to the other treatments. It can be inferred that after the 6th day of incubation, the inhibition of the mycelia of the fungus C. lunata is not satisfactory, since the third assessment at 5 mg mL−1 does not differ from that at 2.5 mg mL−1, and this also does not differ from the positive control (Table 2). The last two assessments (8 and 10 days) confirm that 2.5 and 5 mg mL−1 are statistically equivalent to the mycelial growth of the positive control.

For the inhibitory effect, the concentration 7.5 mg mL−1 can be used, since in all the five assessments, there was a reduced growth of fungal mycelia for the same concentration, and for 10 and 50 mg mL−1, lower quantity of oil will be required. On the other hand, the application of higher concentrations can be more efficient because the pathogen can be inhibited more quickly (Table 2). For C. lunata, the first negative control with methyl thiophanate showed no significant difference in the positive control, even with the use of the concentration above the recommended dose. On all days of incubation, the fungus exhibited growth with the same measurements. The effect of the concentrations of the essential oil of L. sidoides (0, 0.625, 1.25, 2.5, and 5 mg mL−1) was also found in the conidial germination as shown in Figure 3. There was inhibition of conidial germination at increasing doses of L. sidoides essential oil. The two highest concentrations completely inhibited the conidial germination. At the concentration of 2.5 mg mL−1, the conidia germinated in 7% of the total, producing an inhibition of 93%, whereas at the concentrations of 0.625 and 1.25 mg mL−1, inhibitions of 98.83 and 60.17% were observed, respectively (or 1.17 and 39.83% of conidial germination, respectively). The lowest concentration refers to the positive control, which provided a suitable environment for the conidial germination at 100%. Thus, the concentration of 2.5 mg mL−1 was the one that showed better results at 24 h of analysis. A negative control with methyl thiophanate (1 mg mL−1) was used, where it was observed that there was also a complete inhibition of conidial germination of C. lunata.

The phytotoxicity diseases in maize plants have as their primary symptom the appearance of necrotic spots on the leaves, specifically in the regions where the solution builds up, such as on the edges and in the leaf ribs causing loss of leaf green area (Magalhães et al., 2000). In the phytotoxicity assay, the same concentrations were used in the on mycelia C. lunata. Symptoms developed 12 h after the applications of the essential oil in the leaves. It was verified that the concentrations of 10 (10.67% of phytotoxicity) and 50 mg mL−1 (69.33% of phytotoxicity) may not be used for tests of disease control in the plant, because they resulted in wilting, followed by subsequent necrosis in some regions. Based on these results, it can be inferred that the solutions of the oil must be used at a concentration below 7.5 mg mL−1, where there were no visible symptoms of phytotoxicity developing from the application of the essential oil studied. Regarding the preventive effect of L. sidoides essential oil under the progression of the disease spot of Curvularia (Figure 4, black column), the plants treated with the fungicide methyl thiophanate showed symptoms of the disease in smaller areas than those with all other treatments.
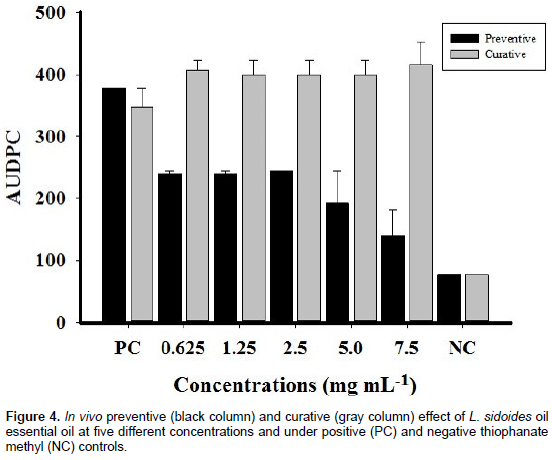
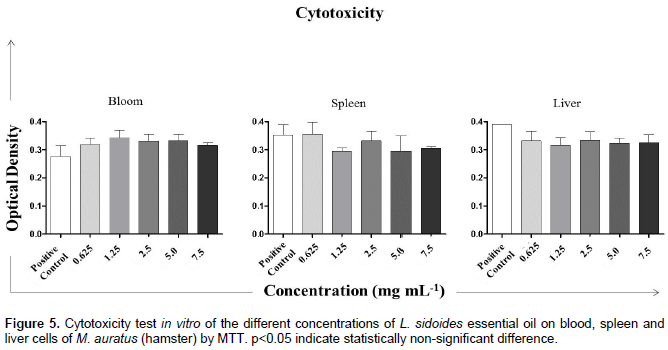
However, it is obvious that there is a large difference between the progression of the disease in the positive control (Tween 80% (0.03%) in sterile distilled water or 1 = 0.0 mg mL−1) and that with other treatments. The black columns in Figure 4 indicate the approximate area values for the concentrations 0.625, 1.25, and 2.5 mg mL−1. At the concentration 5 mg mL−1, a decline in the progression of the disease was observed and when the leaf area with disease at the concentration of 7.5 mg mL−1 is observed (Figure 4), one may say that the value is very close to the negative control. In cytotoxicity assay, L. sidoides essential oil at concentrations of 0.625, 1.25, 2.5, 5.0, and 7.5 mg mL−1 was not toxic to any of the cells tested (Figure 5). It was observed that the values of optical density were very close to all substances, where the control used was sterile distilled water. The amount of formazan, measured by spectrophotometry, was directly proportional to the number of viable cells. Shortly, the cells were metabolically active, both in the presence of five concentrations of the essential oil and in the presence of sterile distilled water.
Different values were reported by Veras et al. (2014), where they obtained 84.9% concentration of thymol and 5.33% of ρ-cymene in essential oil from L. sidoides. Fontenelle et al. (2007) also found much lower values of 59.65% of thymol. Botelho et al. (2007) analyzed the essential oil of L. sidoides and reported a lower concentration (56.7%) of the major compound thymol. These monoterpenes, according to Shettigar et al. (2015) can be found in several species of Lippia. It was observed in the present study that this plant has a great potential to be used in the alternative control of phytopathogenic fungi, since it is possible to obtain high concentrations of the major compound. Thymol has been recognized as a compound that has antimicrobial, insecticidal, leishmanicidal, larvicidal, acaricidal, and anti-inflammatory activities (Santos et al., 2015; Carvalho et al., 2013). Cavalcanti et al. (2004) found a concentration of 80.8% of thymol in the essential oil from plants of this species. The strong effect of thymol from the essential oil of Lippia was also verified by Fontenelle et al. (2007) on the growth of the fungal species Microsporum canis, Malassezia pachydermatis, and Candida species with inhibition of 100% of the mycelia, evidencing the fungistatic activity of the compound thymol. Furthermore, the essential oil and thymol compound have a reducing effect on the CFU in biofilms of Enterococcus faecalis in vitro (time of maturation 72 h), with an exposure time of 30 and 60 min at concentrations of 2.5 and 10%.
These results show that the concentration 50 mg mL−1 inhibited the mycelial growth at 100% (Figure 2). Similarly, Souza Júnior et al. (2009) showed the inhibition of mycelial growth of the phytopathogenic fungus Colletotrichum gloeosporioides, causing the disease anthracnose in the yellow passion fruit, by 100% at concentrations of 1, 3, 5, and 10 mg mL−1 using the essential oil of L. sidoides when compared with that in the positive control (0 mg mL−1). Based on this result, it is concluded that the active principle used was not effective in controlling this pathogen. On the other hand, in the second negative control, mycelial inhibition of Fusarium genus was observed when subjected to the same fungicide and at the same concentration. Concentration above the recommended dose (2 mg mL−1) inhibited, at the end of 10 days, growth with a percentage of inhibition of 88.32% (Table 2). A strong inhibition of the formation of germ tube of the conidia was also observed. This sharp reduction is due to effect of the contact components, induced by essential oils, on the development of the mycelia. It can be inferred that the fungitoxic substance may have acted on the transduction signals involved in the change from the vegetative stage to the reproduction stage (Kumar et al., 2014). Regarding the curative effect (Figure 4, gray column), it was observed that L. sidoides essential oil showed no significant results. All five concentrations used practically had the same area.
In addition, the areas of these treatments were higher than those with the treatment with positive control (PC). Analyzing the column NC (negative control), where there was the presence of the fungicide methyl thiophanate, the progression of the disease was less, and comparing it with the black column NC for the preventive effect (for the same negative control), there was no difference. It can be suggested that it is indifferent to apply the fungicide before or after the occurrence of the disease, because the effect will be the same. However, for L. sidoides essential oil, it was observed that the same can be used as an alternative control, but only by applying the treatments in a preventive manner, that is, prior to the occurrence and progression of the disease. There are no reports in the scientific literature on the preventive and curative effects regarding any essential oil on the disease spot of Curvularia. However, Vigo et al. (2009) showed good results for the effect of the dye of L. sidoides on the occurrence of symptoms (AUDPC of the common bacterial blight) on leaves of string beans Bragança cultivar inoculated with Xanthomonas axonopodis pv. phaseoli, in three application times of the dye (5 days before, 5 days before + 5 days, 5 days after the inoculation of bacteria). The dye had an effect in controlling the disease, with concentrations of 5, 10, and 20% providing the lowest values of AUDPC.
Nagy et al. (2014) reported the preventive and curative effects of the essential oil of cinnamon (Cinnamomum verum). The concentration 0.2% was protective and curative against Venturia inaequalis when applied 24 h before, 1 h before, and 24 after the inoculation. Determining and understanding the mechanisms of action of thymol is a great need with regard to pathogenic fungi. It is known that due to the presence of the group -OH in the molecular structure, this major compound of L. sidoides essential oil has the ability to connect to the amine groups and hydroxylamine of proteins present in cell membranes. Consequently, there is a release of cell contents through changes in the permeability of the fungal membranes (Juven et al., 1994). Regarding the preventive effect, the conidia were inoculated 1 h after the treatment with the essential oil. This means that the compounds in the substance studied might have a greater contact with the fungal conidia in the imminence of the formation of the germ tube and the subsequent development of hyphae. It can be just that thymol altered the permeability of the cytoplasmic membrane of C. lunata, resulting in the inhibition of the biosynthesis of ergosterol content in a manner similar to that of fluconazole (Ahmad et al., 2011), as observed in the membranes of conidia of Candida species.
It can also be said that the essential oil of L. sidoides probably has a protective mechanism of action, that is, the toxic substances are absorbed by the fungus cell, usually through the germ tube (Nene and Thapliyal, 1979). Thus, when the inoculum (conidia) is deposited in the susceptible tissues and germinates, the germ tube comes into contact with the toxic compound (in this case, the essential oil), absorbing it, which later determines, through biochemical mechanisms, the death of protoplasm. The residual action aims to avoid penetration, thus preventing the infection that would occur in the future. Therefore, essential oils that leave residues act by preventing or decreasing the rate of penetration of the pathogen into host tissues, thereby reducing the number of penetrations or future lesions. The fungicide used, methyl thiophanate, is considered to be a systemic type, which exhibits a longer protective action due to its ability to be redistributed within organs treated with acropetal and basipetal translocation (Marsh, 1977). In addition, it does not require germination of conidia as the type of protector. Therefore, it was effective in both the preventive and curative effects of the progression of the disease. Probably, the curative effect of L. sidoides essential oil was not verified due to nonpenetration of the oil into the cell wall of the fungus already inoculated into the plant.
In the present study, the cytotoxic effect of different concentrations of L. sidoides essential oil on blood, spleen, and liver cells of M. auratus (hamster) was also verified. This was necessary to verify if the concentrations used in the maize plants bioassay could be toxic to animals cells, since a compound considered as an alternative control should not cause harm to the people who manipulate it. Therefore, the cell viability test through MTT assay is a method that is based on the ability of metabolically viable cells to reduce the salt of MTT. MTT is a compound soluble in water, which in solution features a pale coloration, being easily incorporated by viable cells. The cells reduce this compound in their mitochondria using the succinic dehydrogenase enzyme. Dehydrogenases are associated with NADPH and NADH. When reduced, MTT is converted into crystals of formazan, a dark-blue compound, not soluble in water and stored in the cytoplasm (Mosmann, 1983). Essential oils can gain a broader focus on this issue and are being demonstrated as potential tools to help prevent diseases, including their antimicrobial activity. In this study, the antifungal activity of the essential oil of the plant species L. sidoides Cham. (Rosemary pepper) was determined, which indicated that this plant has a great antifungal potential against C. lunata.
The authors declare that there is no conflict of interests regarding the publication of this paper.
The authors thank the Federal University of Tocantins, Graduate Agronomy and Biotechnology, and Bioprocess Engineering Departments. This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
|
Adams RP (2007). Identification of essential oil components by gas chromatography/mass spectrometry, fourth ed. Carol Stream: Allured pub.
|
|
|
|
Agrios GN (2005). Plant pathology, fifth ed., Amsterdam: Elsevier Academic Press
|
|
|
|
|
Aguiar RWS, Ootani MA, Ascencio SD, Ferreira TPS, Santos MM, Santos GR (2014). Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Scientific World J.
Crossref
|
|
|
|
|
Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan LA, Manzoor N (2011). Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 30:41-50
Crossref
|
|
|
|
|
Archer DB, Connerton IF, Mackenzie DA (2008). Filamentous fungi for production of food additives and processing aids. Adv. Biochem. Eng. Biotechnol. 111:99-147
Crossref
|
|
|
|
|
Balbi-Pe-a MI, Becker A, Stangarlin JR, Franzener G, Lopes MC, Schwan-Estrada KRF (2006). Controle de Alternaria solani em tomateiro por extratos de curcuma longa e curcumina – I. avaliação in vitro. Fitopatol. Bras.31:310-314
Crossref
|
|
|
|
|
Bennett JW (1998). Mycotechnology: the role of fungi in biotechnology. J. Biotechnol. 66:101-107
Crossref
|
|
|
|
|
Botelho MA, Nogueira NAP, Bastos GM, Fonseca SGC, Lemos TLG, Matos FJA, Montenegro DJ, Heukelbach VSR, Brito GAC (2007). Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J Med Biol Res. 40:349-356
Crossref
|
|
|
|
|
Carvalho RRC, Laranjeira D, Carvalho Filho JLS, Souza PE, Blank AF, Alves PB, Jesus HCR, Warwick DRN (2013). In vitro activity of essential oils of Lippia sidoides e Lippia gracilis and their major chemical components against Thielaviopsis paradoxa, causal agent of stem bleeding in coconut palms. Quim Nova. 36:241-244.
Crossref
|
|
|
|
|
Cavalcanti ESB, Morais SM, Lima MAA, Santana EWP (2004). Larvicidal activity of essential oils from brazilian plants against Aedes aegypti L. Mem Inst Oswaldo Cruz. 99:541-544
Crossref
|
|
|
|
|
Fancelli AL (1988). Fenologia do milho, Piracicaba: ESALQ/USP
|
|
|
|
|
Ferreira DF (2001). Sistema de análises de variância para dados balanceados, SISVAR 4.6, UFLA, Lavras, Brazil
|
|
|
|
|
Ferreira LS (2010). Caracterização de isolados de Curvularia ssp. endofíticos de milho (Zea mays L.) por parâmetros morfológicos e moleculares. Dissertation, Federal University of Paraná
|
|
|
|
|
Fontenelle ROS, Morais SM, Brito EHS, Kerntopf MR, Brilhante RSN, Cordeiro RA, Tomé AR, Queiroz MGR, Nascimento NRF, Sidrim JJC, Rocha MFG (2007). Chemical composition, toxicological aspects and antifungal activity of essential oil from Lippia sidoides Cham. J. Antimicrob. Chemother. 59:934-940
Crossref
|
|
|
|
|
Freitas SP, Moreira JG, Freitas ILJ, Freitas Júnior, SP, Amaral Júnior, AT, Silva VQR (2009). Fitotoxicidade de herbicidas a diferentes cultivares de milho-pipoca. Planta Daninha 27:1095-1103.
Crossref
|
|
|
|
|
Gao S, Liu T, Li Y, Wu Q, Fu K, Chen J (2012). Understanding resistant germplasm-induced virulence variation through analysis of proteomics and suppression subtractive hybridization in a maize pathogen C. lunata. Proteomics 12:3524-3535.
Crossref
|
|
|
|
|
Guimarães LGL, Cardoso MG, Zacaroni LM, Lima RK (2008). Influência da luz e da temperatura sobre a oxidação do óleo essencial de capim-limão (Cymbopogon citratus (D.C.) Stapf). Quim. Nova 31:1476-1480.
Crossref
|
|
|
|
|
Hou JM, Ma BC, Zuo YH, Guo LL, Gao SG, Wang YY, Liu T (2013). Rapid and sensitive detection of C. lunata associated with maize leaf spot based on its Clg2p gene using semi-nested PCR. Lett. Appl. Microbiol. 56:245-250.
Crossref
|
|
|
|
|
Juven BJ, Kanner J, Schued F, Weisslowicz H (1994). Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 76:626-631.
Crossref
|
|
|
|
|
Kern Me, Blevins KS (1999). Micologia Médica: texto & atlas, second ed. São Paulo: Premier.
|
|
|
|
|
Krawczyk N, Armando M, Fonseca M, Lima J (2017). Suicide mortality among agricultural workers in a region with intensive tobacco farming and use of pesticides in Brazil. J. Occup. Environ. Med. 56:993-1000.
Crossref
|
|
|
|
|
Kumar V, Mathela CS, Tewari G, Bisht KS (2014). In vitro inhibition activity of essential oils from some Lamiaceae species against phytopathogenic fungi. Pestic. Biochem. Physiol. 114:67-71.
Crossref
|
|
|
|
|
Magalhães PC, Da Silva JB, Durães FOM (2000). Fitotoxidade de herbicidas aplicados em pós-emergência na fase inicial da cultura do milho. Planta Daninha 18:277-284.
Crossref
|
|
|
|
|
Marsh RW (1977). Systemic fungicides. second ed. London: Longman 401 p.
|
|
|
|
|
Milanesi PM, Blume E, Muniz MFB, Brand SC, Junges E, Manzoni CG, Weber MND (2009). Ação fungitóxica de extratos vegetais sobre o crescimento micelial de Colletotrichum gloeosporioides. Rev. FZVA. 16:01-13.
|
|
|
|
|
Mosmann T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63.
Crossref
|
|
|
|
|
Mühlen CV (2009). Índices de retenção em cromatografia gasosa bidimensional abrangente. Sci. Chromatogr. 1:21-29.
|
|
|
|
|
Muthaiyan A, Martin EM, Natesan S, Crandall PG, Wilkinson BJ, Ricke SC (2012). Antimicrobial effect and mode of action of terpeneless cold-pressed Valencia orange essential oil on methicillin-resistant Staphylococcus aureus. J. App. Microbiol. 112:1020-1033.
Crossref
|
|
|
|
|
Nagy G, Hochbaum T, Sárosi S, Ladányi M (2014). In Vitro and in Planta activity of some essential oils against Venturia inaequalis (Cooke) G. Winter. Not. Bot. Hortic. Agrobot. 42:109-114.
|
|
|
|
|
Nene YL, Thapliyal PN (1979). Fungicides in plant disease control. Second ed. New Delhi: Oxford & IBH Publishing 507 p.
|
|
|
|
|
Okumura RS, Mariano DC, Zaccheo PVC (2011). Uso de fertilizantes nitrogenados na cultura do milho: uma revisão. PA&T. 4:226-244.
|
|
|
|
|
Reis EM, Casa RT, Bresolin ACR (2004). Manual de diagnose e controle de doenças do milho. Lages: Graphel 44 p.
|
|
|
|
|
Riss TL, Moravec RA, Niles AL, Duellman S, Benink HA, Worzella TJ, Minor L (2013). Cell Viability Assays. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences.
View Accessed 01 May 2016.
|
|
|
|
|
Sá DAC, Santos GR, Furtado GQ, Erasmo EAL, Nascimento IR (2011). Transport, pathogenicity and transmissibility of fungi associated with physic nut seeds. RBS. 33:663-670.
|
|
|
|
|
Santos CP, Oliveira TC, Pinto JAO, Fontes SS, Cruz EMO, Arrigoni-Blank MF, Andrade T, Matos IL, Alves PB, Innecco R, Blank AF (2015). Chemical diversity and influence of plant age on the essential oil from Lippia sidoides Cham. Germplasm. Ind. Crops Prod. 76:416-421.
Crossref
|
|
|
|
|
Santos GR, Brum RBCS, Castro HG, Gonçalves CG, Fidelis RR (2013). Effect of essential oils of medicinal plants on leaf blotch in Tanzania grass. Rev. Cienc. Agron. 44:587-593.
Crossref
|
|
|
|
|
Santos GR, Café-Filho AC, Leão FF, César M, Fernandes LE (2005). Progresso do crestamento gomoso e perdas na cultura da melancia. Hortic. Bras. 23:228-232.
Crossref
|
|
|
|
|
Schneider RW, Williams RJ, Sinclair JB (1976). Cercospora leaf sport of cowpea: models for estimating yield loss. Phytopathology 66:384-388.
Crossref
|
|
|
|
|
Seixas PTL, Castro HG, Santos GR, Cardoso DP (2011). Controle fitopatológico do Fusarium subglutinans pelo óleo essencial do capim-citronela (Cymbopogon nardus L.) e do composto citronelal. RBPM (Impresso) 13:523-526.
Crossref
|
|
|
|
|
Shettigar NB, Das S, Rao NB, Rao SBS (2015). Thymol, a monoterpene phenolic derivative of cymene, abrogates mercury-induced oxidative stress resultant cytotoxicity and genotoxicity in hepatocarcinoma cells. Environ. Toxicol. 30:968-980.
Crossref
|
|
|
|
|
Souza Júnior IT, Sales NLP, Martins ER (2009). Efeito fungitóxico de óleos essenciais sobre Colletotrichum gloeosporioides, isolado do maracujazeiro amarelo. Biotemas 22:77-83.
|
|
|
|
|
Sun R, Gao YX, Shen KZ, Xu YB, Wang CR, Liu HY, Dong JY (2010). Antimicrobial metabolites from the aquatic fungus Delitschia corticola. Phytochem. Lett. 4:101-105.
Crossref
|
|
|
|
|
Vaz-De-Melo A, Afférri FS, Dotto MA, Peluzio JM, Santos GR, Carvalho EV (2010). Reação de híbridos de milho à Curvularia ssp, sob dois níveis de adubação com nitrogênio, no Sul do Tocantins. Sci. Agrar. 11:149-154.
Crossref
|
|
|
|
|
Veras HNH, Rodrigues FFG, Botelho MA, Menezes IRA, Coutinho HDM, Costa JGM (2014). Antimicrobial effect of Lippia sidoides and thymol on Enterococcus faecalis biofilm of the bacterium isolated from root canals. Sci. World J. 5p.
Crossref
|
|
|
|
|
Vigo SC, Maringni AC, Camara RC, Lima GPP (2009). Ação de tinturas e óleos essenciais de plantas medicinais sobre o crestamento bacteriano comum do feijoeiro e na produção de proteínas de indução de resistência. Summa Phytopathol. 35:293-304.
Crossref
|
|
|
|
|
Zolan ME, Pukilla PJ (1986). Inheritance of DNA methylation in Coprinus cinerus. Mol. Cell Biol. 6:195-200.
Crossref
|
|