ABSTRACT
A cross sectional study design was used to determine the prevalence and species spectrum of major gastrointestinal parasites affecting camels; and to find out risk factors associated with this parasitic infestation in Yabello district, southern rangelands of Ethiopia. A total of 412 camels of all age and sex were examined between August, 2011 and March, 2012. Collected faecal samples were processed by standard floatation methods and then examined for helminth eggs. Coprological examination revealed that 73.8% (n=304) of the camels excreted helminth eggs/protozoan oocyst in their faeces. Six types of helminth/protozoan parasites eggs/oocyst encountered in descending order of prevalence were, Strongylus species 55.59%, Strongyloides species 13.82%, Trichostrongylus species 10.19%, Monezia species 6.91%, Coccidia and Trichuris species each encountered 1.32%. Single and concurrent infections with two or more parasites were recorded in 89.15% and 10.85% of the cases, respectively. Except for age and treatment factors significantly affected (P<0.05) the prevalence of gastrointestinal parasite infections, all the other factors like origin, sex, body condition score and health status have shown no significant effect on parasitic infestation. The high prevalence and wide spectrum observed in the present study suggests that helminth infection are widespread and may be a constraint to economic camel production, and there is need to institute control measures.
Key words: Camels, gastrointestinal parasites, prevalence, risk factors, Yabello.
Camels are important multipurpose animals of arid and semi-arid parts of the world. Camel is a very hardy animal and anatomically as well as physiologically well adapted to harsh climatic conditions of desert areas of the world, including Ethiopia. Camel is the most important livestock that can live and produce in poor farms, and can be compared with high-yield animals of the same weight, like cattle, in productivity under manual feeding. Hence, there is a need to improve management of camels considering its prospect in the semiarid and arid regions where livestock production is becoming more difficult due to climate changes (Sazmand, 2011).
Camels suffer from various internal and external parasitic diseases which are major causes of impaired fertility and low calving rates of camels as well as impaired milk and meat production. Moreover, parasitic diseases may also predispose them to other infections, lower the working efficiency or result in death, and sometimes serve as potential danger for public health (Anwar and Khan, 1998). With the introduction of sedentary, semi-intensive camel farming systems, parasites may assume much more significant role in camel husbandry (Parsani et al., 2008).
Camels can acquire helminth infection by grazing on infected pastures or by ingesting infective larvae with drinking water. Signs and symptoms of gastrointestinal helminths in camels are numerous, mainly weight loss along with growth disorders, colic, fever, diarrhoea, anemia, gastritis and enteritis (Fowler, 1996). However, the clinical manifestations of helminthosis may be subclinical or asymptomatic, in which case the animal appears normal but performs below its full potential (Borji et al., 2010).
Despite being usually reared under harsh environments unsuitable for propagation and transmission of helminths, camels are capable of harbouring a fairly large variety of internal parasites. There is paucity of literature as helminthic infections of camels are generally regarded less of a problem than those in other ruminants. Among others, the camel stomach worm Haemonchus longistipes is the most pathogenic strongyle nematode of camels. Trichostrongyles are also very common and may contribute to the debilitating effects of gastrointestinal nematodes. Extraintestinal nematodes commonly parasitizing camels include Onchocerca fasciata, which characteristically produces subcutaneous nodules in the head and neck regions; the filarial worm Dipetalonema evansi, the eye worm Thelazia and rarely the lungworms (Dictyocaulus or Protostrongylus species). Among larval cestodes, hydatid cysts are commonly reported, while Cysticercus and Coenurus species are infrequent (Chhabra and Gupta, 2006).
Climate plays a dominant role in determining the timing and size of peak larval contamination on pasture (Suolsby, 1982). Various studies have shown a relationship between the onsets of parasitic gastroenteritis and meteorological data (Thomas and Starr, 1978). Accordingly, Haemonchus and Oesophagostomum colombianum predominate in hot climate while Trichostrongylus and Ostertagia and Oesophagostomum venulosum are predominate in warm climates (Levine, 1978).
Currently, there is growing awareness of the unique role that camel plays in the cultural heritage and socio-economics in Ethiopia. The increasing value of the camel, however, has verified the economic viability of health care. The most important is that, camel can tolerate harsh conditions and thrive better if good control means for the diseases affecting camels are adopted. Recently drought has become increasing and brings great challenges on livestock production in pastoral areas of Borena which increase importance of camel production in the area. Data on gastrointestinal parasites of camel are less available in pastoral area of Borena zone. Hence, the present study is very important to give base line data on gastrointestinal parasites of camel which improve their production and reproduction. Therefore, a questionnaire and parasitological survey was carried out in camel herds in different localities of Yabello district, in order to obtain information on the relationship between various host factors and to estimate the prevalence and identify the species spectrum of major gastrointestinal parasites affecting camels in Yabello district, southern rangelands of Ethiopia.
Description of study area
The study was conducted in Yabello district of Oromia regional state, which is found at about 575 km south of Addis Ababa. According to data from the district and vaccination survey of zonal veterinary service of 2010/2011, Yabello has a livestock population of 435,553; of which camels are estimated at 22,972. Delivery of the rainfall is bimodal: 56% of the annual rainfall occurs with long rains expected from March to May and 27% the short rains from mid September to mid November (Coppock, 1994). The area has a migratory route of livestock during drought and animal trading from it neighboring country, regions and districts. Hence, huge livestock population and encroachment of bushes in the area brought shortage of livestock feed, increase movement and disease transmission and difficulty of livestock disease control.
Study animal
The study animals consisted of indigenous breeds of camels (one hump camel) reared under extensive management system which allows free grazing, usually mixed with livestock from other villages, and in which the animals move from feed shortage area to those improved with feed intake especially during drought season. During sampling gender, age, body condition, treatment history and the presence of clinical signs were recorded.
Study design and sampling method
A cross sectional study to estimate the prevalence of gastrointestinal parasites infestation and stratified random sampling techniques were used to collect the data between August, 2011 and March, 2012. Risk factors like body condition, gender, sex, history of treatment and clinical health status most probably associated with parasitic disease was collected at the time of sampling using structured (closed) questionnaires. Out of 23 kebeles of Yabello district, six were selected by considering accessibility and our facilities. From the selected localities, households were randomly selected. Camels from each selected household of localities were examined with proportional sample size of the total camel population from each kebele. Accordingly, 108, 42, 80, 48, 50 and 84 camels were selected from Surupha, Bake, Dide-Hara, Dherito, Haro-wayu and Areri kebeles, respectively.
Sample size determination
The desired sample size for the study was calculated using the formula given by Thrusfield (2005) with 95% confidence interval, at 5% precision and by assuming maximum 50% expected prevalence of camel gastrointestinal parasite infestation in the area. The calculated sample size was 384, but to increase the precision of sampling in the study, 412 camels were considered.
Study methodology and parasitological examination
The gender, body condition score based on http://www.camelsaust.com.au/livebodycond.htm and further classified as poor (score 1 and 2), medium (score 3) and good (score 4 and 5), age group (<4 years, 4-6 years and >6 years), the health condition (apparently healthy and camels with any signs (emaciation, depression, intermittent diarrhea, milk production and weight losses, coughing and nasal discharge) and deworming history (dewormed and non-dewormed camels) were considered during the study. Fresh fecal samples were collected per rectum from individual camel using plastic gloves, put into faecal pots, labelled and immediately transported to Yabello Regional Veterinary Diagnostic Laboratory. Individual samples were processed by using standard flotation techniques as described by Hansen and Perry (1994). All parasite eggs were identified morphologically as described by Soulsby (1982), Boid et al. (1986), Urquhart et al. (1996) and Max et al. (2006).
Closed type questionnaire survey was also carried out to interview individual owners to obtain general information about camel age, previous anthelmintics administration and appearance of any clinical signs/syndromes.
Data management and statistical analysis
All collected data was entered to MS excel sheet and analyzed by using SPSS version 19. Descriptive statistics was used to determine the prevalence of the parasites and the risk factors associated to the disease (age, sex, body condition, health status and history of deworming) was related using Chi-square test (χ2) for their significant difference by using confidence level at 95% and P<0.05 for significance.
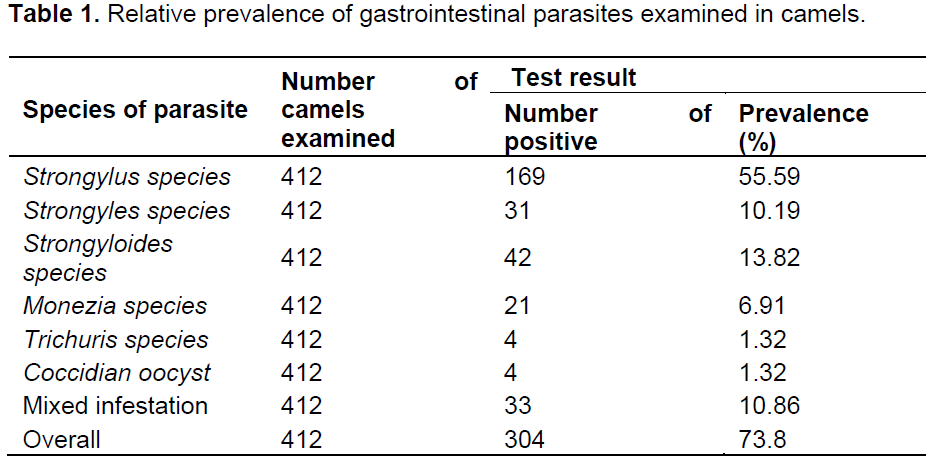
Of the total 412 camels examined, 304 camels (73.8%) were observed to harbor one or more types of gastrointestinal parasites at varying levels. As shown on Table 1, the identified parasite includes different nematode species, cestodes (monezia species) and coccidian oocyst.
Gastrointestinal parasite prevalence variation with host related risk factors
The overall gastrointestinal parasite infestation in different age groups and health status of camel were revealing statistically significant variation among camels in different age groups (X2=6.73; P<0.05) and health status (X2=4.95; P<0.05). However, gastrointestinal parasite infestation in relation to camel sex and body condition showed no statistical significant variation (P>0.05) in both cases (Table 2).
Gastrointestinal parasite infestation variation with anthelmintics usage
The analysis of questionnaire survey from camels owner revealed the effect of anthelmintics usage on the prevalence of gastrointestinal parasite infestation and the variation was found to be statistically significant (X2=47.78; P<0.05) (Table 2).
Gastrointestinal parasite prevalence variation with origin of camels
The data analysis conducted during the study indicated no of significant association (X2=5.58; P>0.05) between
camels origin and gastrointestinal parasite infestation (Table 3).
It is evident from the results of this study that helminthosis was an important health disease in camels. This finding is in agreement with the results of other researchers, that helminthosis is one of the main problems in camel worldwide (Selim and Rahman, 1972; Fadl et al., 1992; Abdul-Salam and Farah, 1988; Rewatkar et al., 2009; Khan et al., 2010). According to our results, 73.8% (304/412) of camels harboured at least one type of gastrointestinal parasite eggs/oocyst. This finding almost coincides with previous report of overall infection rates of 75% in Eastern Ethiopia (Bekele, 2002), 75.1%, in Iranian camels (Borji et al., 2010), and 76.2% in Bahrain (Abubakr et al., 2000). However, it is lower than prevalence reports of Sharrif et al. (1997) in Jordan; Tekle and Abebe (2001) in Ethiopia; Bamaiyi and Kalu (2011) in Nigeria; Demelash et al. (2014) in Yabello district of Ethiopia, who reported prevalence of 98, 96.92, 92.4 and 80.73%, respectively. In contrast, lower rate of 68.9 and 62.7% reported from dromedaries in Nigeria (Kamani et al., 2008) and in Northern Tanzania (Swai et al., 2011), respectively. The possible explanation for the country to country variation in the infestation rate could be variations in agro-ecological conditions between countries, which favor or disfavor the survival of parasites eggs or larvae, levels of hygiene and husbandry practices (Allport et al., 2005). Moreover, the occurrence of parasite is associated with nutritional status, level of immunity, rainfall, humidity and temperature differences and season of examination on the respective study areas.
Six different types of gastrointestinal worms and protozoan were identified in camels. They were broadly classified as nematodes (4 species), cestode (1 species) and protozoan (1 species) according to the egg/oocyst structure (Soulsby, 1982; Boid et al., 1986, Urquhart et al., 1996; Max et al., 2006). Mixed parasitism (10.85%) involving two or more helminths and protozoan genera was common in the present study and is in agreements with the results of other researchers (Bekele, 2002; Rewatkar et al., 2009; Swai et al., 2011). Strongylus species occurred in 169 of 412 camels (55.59%) screened and was the most prevalent gastrointestinal parasite encountered during the study. This prevalence was comparable to the prevalence of 41% reported in Ethiopia (Bekele, 2002), but lower than the prevalence’s of 100, 89.2 and 75% reported in Kenya, Northern Tanzania and Sudan, respectively (Mukani and Kimani, 1999; Swai et al., 2011; Abdul-Salam and Farah, 1988). The relatively high level of gastrointestinal parasitism recorded in this study is probably related to the number of adult parasites established in the gastrointestinal tract, level of host immunity, stage of parasite infection, and lack of improvement in animal health management programmes or non adoption of the modern animal health care programmes by camel owners.
Eimeria species with prevalence of 1.32% was low compared with prevalence of 9.9, 12.5 and 25% respectively recorded in northern Tanzania and Pakistan (Swai et al., 2011; Anwar and Khan, 1998; Rewatkar et al., 2009). Heavy protozoan infection causes significant impact in young camels resulting into high morbidity and mortality (Chineme, 1980; Boid et al., 1986; Kinne and Wernery, 1997).
Significant factors might influence the prevalence of gastrointestinal parasites infestation. Host age was found to be a significant factor with respect to gastrointestinal parasite infection (P<0.05), with eggs/oocyst been detected more frequently in age categories >6 years than 4 to 6 years and <4 years camels. Camels reported to have been treated against helminths in the last one year prior to the study survey were significantly infected (p<0.05) by gastrointestinal parasites than untreated camel. Moreover, males were more likely to harbour gastrointestinal parasites eggs/oocyst than female camels, but the variation was not significant (P>0.05).
The study further revealed that health status, origin and body condition of the camel did not show significant association (P>0.05) with the prevalence of parasite infestation. The absence of association between body condition and prevalence disagrees with previous reports of Swai et al. (2011) and studies in other livestock species (Keyyu et al., 2003). This could be explained by the fact that loss of body condition in the camels could be due to other factors, such as seasonal change of forgeable feed and presence of other concurrent disease conditions, mainly high prevalence of trypanosomosis in some of the lowland areas.
In conclusion, as most of the gastrointestinal helminth species in camels are also common to cattle, sheep and goats, strategic deworming of camel using broad spectrum anthelmintics seems necessary for enhancing productivity of camels as well as other livestock kept near them. Moreover, from the results of this study parasitism is one of the major health problems of camels which need special attention to save the already poor people from poverty who are the main camel keepers in Yabello, Ethiopia. These people use camels for carriage purposes as well as a source of milk and meat in addition to their use as draught animals. For this purpose, it is suggested that livestock disease diagnostic and monitoring centers be strengthened in the area to look after the health, management and breeding aspects of the camels.
The authors have not declared any conflict of interest.
The authors would like to thank Yabello Regional Veterinary Laboratory for financial and logistic support during the study period. We also express our heartfelt thanks to pastoralists of the study settings for their voluntary and active participation in providing information and restraining of the study animals during sampling.
REFERENCES
Abdul-Salam JM, Farah MA (1988). Seasonal fluctuations of gastrointestinal helminths of camels in Kuwait. Vet. Parasitol. 28(1-2):93-102.
Crossref |
|
|
|
Abubakr MI, Nayel MN, Fadlalla ME, Abdelrahman AO, Abuobeida SA, Elgabara YM (2000). Prevalence of gastrointestinal parasites in young camels in Bahrain. Revue d'Élevage et de Médecine Vétérinaire des Pays Tropicaux 53(3):267–271. |
|
|
|
Allport R, Mosha R, Bahari M, Swai ES, Catley A (2005). The use of community-based animal health workers to strengthen disease surveillance system in Tanzania. Revue scientifique et technique International Office of Epizootics 24(3):921-932. |
|
|
|
Anwar AH, Khan MN (1998). Parasitic fauna of camel in Pakistan. Proceedings of the Third Annual Meeting for Animal Production Under Arid Conditions 2:69–76. |
|
|
|
Bamaiyi PH, Kalu AU (2011). Gastrointestinal parasites infection in one humped camels (Camelus dromedarius) of Nigeria. Vet. Res. Forum 2(4):278–281. |
|
|
Bekele T (2002). Epidemiological studies on gastrointestinal helminths of dromedary (Camelus dromedarius) in semi-arid lands of eastern Ethiopia. Vet. Parasitol. 105(2):139-52.
Crossref |
|
|
Boid R, Jones TW, Luckins A G, (1986). The camel in health and disease. 3. Protozoal diseases of camels. British Vet. J. 141: 87-105.
Crossref |
|
|
|
Borji H, Razmi GR, Movassaghi AR, Naghibi A, Maleki M (2010). A study on gastrointestinal helminth of camels in Mashhad Abattoir, Iran. Iranian J. Vet. Res. 11:174. |
|
|
|
Chhabra MB, Gupta SK (2006). Parasitic diseases of camels - an update-2. Helminthoses. J. Camel Pract. Res. 13(2):81-87. |
|
|
|
Chineme CN (1980). A case report of coccidiosis caused by Eimeria cameli in camel (camelus dromedarius) in Nigeria. J. Wild Life. Diseas. 16(3):377-380. |
|
|
|
Coppock DL (1994). The Borana Plateau of Southern Ethiopia. Synthesis of Pastoral Research, Development and Change, 1980-91, ILCA, Addis Ababa, Ethiopia. |
|
|
|
Demelash K, Alemu F, Niguse A, Feyera T (2014). Prevalence of Gastrointestinal Parasites and Efficacy of Anthelmintics against Nematodes in Camels in Yabello District, Southern Ethiopia. Acta Parasitol. Globalis 5(3):223-231. |
|
|
|
Fadl M, Magzoub M, Bürger HJ (1992). Prevalence of gastro-intestinal nematode infection in the dromedary camel (Camelus dromedarius) in the Butana plains, Sudan, Revue d'élevage et de médecine vétérinaire des pays tropicaux., 45(3-4):291-293. |
|
|
|
Fowler ME (1996). Husbandry and diseases of camelids. Revue Rev. Scientifique Sci. Tech. Office Int. Des Epizooties 15(1):155–169. |
|
|
|
Hansen J, Perry B (1994). The epidemiology, diagnosis and control of helminth parasite of ruminants 2 ed. Nairobi, Kenya. |
|
|
|
Kamani J, Turaki AU, Egwu GO, Mani AU, Kida SM, Abdullahi JG, Damina MS, Kumshe HA, Dogo GI (2008). Prevalence of gastrointestinal parasites in Camelus dromedaries slaughtered in Maiduguri, Nigeria. J. Camel Pract. Res. 15(2):181–182. |
|
|
Keyyu JD, Kassuku AA, Kyvsgaard NC, Willingham AL (2003). Gastrointestinal nematodes in indigenous zebu cattle under pastoral and nomadic management systems in the lower plain of Southern highlands of Tanzania. Vet. Res. Comm. 27(5):371-380.
Crossref |
|
|
|
Khan MN, Sajid MS, Khan MK, Iqbal Z, Hussain A (2010). Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol. Res. 107(4):787-94. |
|
|
|
Kinne J, Wernery U (1997). Severe outbreak of camel coccidiosis in the United Arab Emirates. J. Camel Pract. Res. 4:261-265. |
|
|
|
Levine NO (1978). The influence of weather on the bionomics of the free living stages of nematodes in weather and parasitic animal disease, ed. T.E. pp. 117-123. |
|
|
|
Max R, Vatta A, Jayaswal ML, Kimambo AE, Kassuku AA, Mtenga LA (2006). Technical manual for worm management in small ruminants, Sokoine Univerisity of Agriculture, Tanzania. pp. 2-4. |
|
|
|
Mukani O, Kimani K (1999). Efficacy of parenteral formulation of levamisole and ivermectin against Strongylosis in Dromedary Camels. J. Camel Pract. Res. 6(1):73–75. |
|
|
|
Parsani HR, Veer S, Momin RR (2008). Common Parasitic Diseases of Camel Deparment of Parasitology College of Veterinary Science and A.H. S.D. Agricultura University, Sardarkrushinagar, India. Vet. World 1(10):317-318. |
|
|
Rewatkar SG, Deshmukh SS, Deshkar SK, Maske DK, Jurnde PD, Bhangale GN (2009). Gastrointestinal helminthes in migratory camel. Vet. World 2(7):258.
Crossref |
|
|
|
Sazmand A (2011). Prevalence of cryptosporidiosis in camel and camel handlers in Yazd province. Doctor of Veterinary Medicine Thesis, Shahid Chamran University of Ahvaz, Iran. In Persian. pp. 3-5. |
|
|
|
Selim M, Rahman M (1972). Enteric nematodes of camels in Egypt. Egyptian Egypt. J. Vet. Sci. 9:75. |
|
|
|
Sharrif L, Al-qudah KM, Al-Ani FK (1997). Prevalence of gastrointestinal helminths in one-humped camel (Camelus dromedarius) in Jordan. J. Camel Pract. Res. 41:67–69. |
|
|
|
Soulsby EJW (1982). Helminthes, Arthropods and Protozoa of Domesticated Animals, 7th Edition. Bailliere Tindall, London: Lea and Febiger, Philadelphia. pp. 614-615. |
|
|
|
Swai ES, Moshy W, Mshanga D, Lutatina J, Bwanga S (2011). Intestinal parasitic infections of camels in the agro and pastoral areas of northern Tanzania. Veterinary Investigation Centre, Arusha, Tanzania. Vet. Res. 4:34-38. |
|
|
|
Tekle T, Abede G (2001). Trypanosomosis and helminthoses: major health problems of Camels in the southern Rangelands of Borena, Ethiopia. J. Camel Pract. Res. 8(1):39–42. |
|
|
|
Thomas RJ, Starr TR (1978). Forecasting the peak of gastrointestinal nematode infection in lambs. Vet. Res. 103:465-468. |
|
|
|
Thrusfield MV (2005). Veterinary Epidemiology, 3rd ed., Blackwell Science, Oxford, London, UK. pp. 234-238. |
|
|
|
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1996). Veterinary Parasitology, 2nd ed. Black well Science limited, London, UK. P. 307. |