ABSTRACT
This study was conducted on 810 goats in three agro ecological zones (Highland, Midland and Lowland) of Loma district in southern Ethiopia were considered with sex and age groups factor to characterize morphologically Woyto Guji goat types in their home tract. A pre-tested questionnaire was used for recording morphological features, body weights and linear body measurements. Both qualitative and quantitative traits were recorded on randomly sampled goats from three agro ecologies and the data were analyzed using SPSS and SAS software. The goat type in the study area was characterized by higher proportion of plain coat patterns (91.2%) with brown coat color (45.7%), straight head profile (80.6), semi pendulous ear formation (69.8%) and long ear type (97.3%). The horns were characterized by backward orientation with a straight shape. Body weight of the goats’ changes at increasing rate at 0PPI to 3PPI and gradual increase was observed at older ages. Sex, age, agro ecological zones, sex by age and age by agro ecologies interaction had a significant (p<0.05/p<0.01) effect on body weight and many of the linear body measurements. The mean live body weight (BWT), heart girth (HG), height at wither (HW), chest width (CW), pelvic width (PW), rump height (RH), rump length (RL), ear length (EL), and horn length (HL) of females were 26.53±2.91 kg, 57.48±0.64 cm, 70.20±0.21 cm, 64.12±0.18 cm, 13.74±0.07 cm, 13.20±0.19 cm, 66.04±0.52 cm, 11.97±0.13 cm, 13.74±0.16 cm and 11.20±0.10 cm, respectively. The corresponding values for male counterpart were 27.16±0.70 kg, 60.13±1.17 cm, 74.98±0.33 cm, 68.34±0.05 cm, 14.48±0.41 cm, 13.25±0.37 cm, 68.37±0.50 cm, 12.83±0.43 cm, 14.02±0.020 cm and 13.22±0.47 cm respectively. Heart girth had the highest correlation with body weight at various ages and in both sexes compared with other parameters, except in females of zero dentition was not significant. The regression equation for pooled overall age groups was estimated as Y = (-28.20) + 0.74 X; (where X stands for HG), with R2 value of 0.68 for female and Y = (-39.12) + 0.88 X; (where X stands for HG), with R2 value of 0.78 for male goat in the present study. The result indicated that phenotypic characterization, body weight and linear body measurement description could help as an input for efficient utilization, conservation and designing improvement strategy for this genetic resource in the community.
Key words: Age, agro ecology, body weight, linear body measurements, sex.
Ethiopia is home for diverse indigenous goat populations. Studies estimated that about 15 breeds of goat exist in Ethiopia though the goat characterization is not exhaustive (IBC, 2004). Based on phenotypic and molecular characterization, there are four families and 12 different types (FARM-Africa, 1996; Tesfaye, 2004). The goat population of Ethiopia is estimated at 22.78 million heads (CSA, 2011). Goat production is one of the integral parts of livestock farming activities of the country. It has been estimated that about 70% of the goat population is found in the lowlands and the rest 30% is found in the highland Agro ecologies (Alemayehu, 1993, Workneh and Rowlands, 2004). Currently studies revealed that an increasing trend of goat in all agro ecologies (Aschalew et al., 2000).
According to Kiwuwa (1992), the broad genetic variability of African small ruminant breeds enables them to survive under stressful environmental conditions, including high disease incidence, poor nutrition and high temperature. The environmental pressure also maintains a wide range of genotypes, each adapted to a specific set of circumstances. Thus, improvement of local animal genetic resources holds promise for feasible mechanism of conservation through addressing the self-sustaining incentive of improved livelihood for the keepers (Grum, 2010).
Morphological characterization is one of the crucial means for describing the goat breeds. It is essential to characterize a breed for its conservation (Bizhan et al., 2010). Body measurement in addition to weight estimate describe the individual or population than do the conventional methods of weighing and grading small ruminant (Salako, 2006). Body dimensions have been used to indicate breed, origin and relationship through the medium of head measurements (Itty et al., 1997).
The available information of “Woyto-Guji” goat breed was not sufficient to describe the breed and morphological characterization carried so far have not covered all the production environments including Loma districts rather have focused on specific areas of the population besides the information was undertaken before two decades (Workneh, 1992; Farm-Africa, 1996). Indigenous livestock breeds are considered, for diverse reasons, as treasured genetic resources that tend to disappear as a result of new market demands, crossbreeding or breed replacement and mechanized agricultural operations (Halima et al., 2012, Dereje et al., 2013). Therefore, with these all scenarios and the current global animal genetic resource mix up through inbreeding, interbreeding and environmental change it is important to characterize over different agro-ecological zones. The objective of this study was to characterize morphologically the Woyto-Guji goat type in their home areas (Figure 1).
The study was conducted in Loma district, located in Dawuro zone at 6.59° -7.34° N latitude and 36.68° - 37.52° E longitudes with altitudinal range of 501 to 3300 m above sea level in Southern Nations, Nationalities and Peoples Region (SNNPR) (Mathewos, 2008). The district was, selected based on its potential for goat production, diversified Agro ecological zones which encompasses lowland, midland and highland and its varied production system. The total surface area of the district is 116,280ha; with the mean annual rainfall of 900-1800 mm, with bimodal and erratic distribution and temperature ranges from 14 to 30°C (LAR, 2013).
Data collection
Before the commencement of the study, a rapid field survey was conducted by a team of researchers to assess the distribution and population of the goat in different Agro ecology of the study areas. Multi-stage stratified sampling techniques were employed in the present study. In the first stage, district was stratified into three agro ecologies namely lowland with altitude of <1500masl, midland with altitude of 1500-2300 masl and highland with > 2300 masl (MOA, 2000). The Agro ecologies were identified based on altitude and production system of the district. In the second stage, three kebeles were randomly selected from each agro-ecology. In the third stage, a total of 810 goats selected randomly in all direction after every eight to twelve households based on the number of household per each Kebels.
About 12 qualitative characters (head profile, ear formation, ear type, coat color pattern, coat color type, horn shape, horn orientation, ages, presence of wattle, ruff, bear and horn) and 10 quantitative characters, live body weight (BWT), heart girth (HG), height at wither (HtW), chest width (CW), pelvic width (PW), rump height (RH), rump length (RL), ear length (EL), horn length (HL) and scrotal circumference (SC) were collected from a total of 810 goats based on the standard description of the Food and Agriculture Organization of the United Nations list (FAO, 2011). Goats were purposively grouped into 5 age categories based on dentition. These age groups were goats with no pairs of permanent incisors (0PPI) at weaning age below 12 to 14 months, one pair of permanent incisors (1PPI) at age of 15 to 23 months two pairs of permanent incisor (2PPI) at age of 24 to 35 months, three pairs of permanent incisors (3PPI) at age of 36 to 48 months and four pairs of permanent incisors (4PPI) at age of over 48 months (Tatiana, 1999) and sex groups (male and female).
Data analysis
Statistical package for social Science (SPSS) computer software (SPSS ver.20, 2013) was applied to analyze qualitative data. The General Linear Model (GLM) procedures of SAS ver.9.2 were employed to analyze quantitative data and ascertain the effect of sex, site (agro ecology) and age (SAS, 2010). Mean separation was undertaken when it was significant to reveal the difference between means using Tukey-Karamers method.
Yijk = μ + Ai + Sj +Dk + (AS)ij+(AD)ik+(SD)jk+ eijk …… Model 1
Where: Yijk = lth observation on ith production site, jth sex class and kth age group; μ = Overall mean
Ai = Fixed effect of ith Agro ecology (i= 1, 2, 3), Where 1=lowland, 2=midland and 3 = Highland)
Sj = Fixed effect of jth sex (j =1, 2 where 1 = male, 2 = female); Dk =
Fixed effect of kth; dentition (k =1, 2,3,4,5 where 1= 0PPI,2= 1PPI, 3
= 2PPI, 4 = 3PPI and 5 = 4PPI); (AS) ij = fixed effect of interaction between Agro ecology and sex (AD) ik = fixed effect of interaction between Agro-ecology and dentition; (SD)jk =fixed effect of interaction between sex and dentition, eijk = random error;
Correlations (Pearson’s correlation coefficients) between body weight and different linear measurements were computed for the population within each sex and dentition categories.
The stepwise REG procedures of SAS ver.9.2 was used to predict live weight from body measurements for pooled data, separate sexes and for each age categories (SAS, 2010). The choice of the best fitted regression model was selected by using coefficient of determination (R2) and Mean standard error (MSE).
Yj = β0 + β1X1 + β2X2 + β3X3 + β4X4 + β5X5 + β6X6+ β7X7+ β8X8 + β9X9+ β10X10+ej Model 2 (Female)
Yj = β0 + β1X1 + β2X2 + β3X3 + β4X4 + β5X5 + β6X6+ β7X7+ β8X8+ β9X9+ β10X10+ β11X11+ ej ……. Model 3 (Male)
Goat population characterization
The average age of different categories of goats in terms of the eruption of permanent pairs of incisors (PPI) was assessed. The present study revealed that the average ages of goat with 0PPI, 1PPI, 2PPI, 3PPI and 4PPI were around 9±4.12, 18.4±3.19, 30.11±6.98, 41.4±8.86 and 49.83±12.55 months, respectively. The result in the current study was comparable with earlier study indicated 7±2.12, 16.4±4.19, 27.11±5.98 and 38±6.86 and 50.83±14.55 months, respectively in Harrarghe highland goats (Dereje et al., 2013). The variation of eruption of incisors and corresponding age could be caused due to variation in breed, environment, feeding habit and production system (Table 1).
Qualitative characteristics
The participatory descriptions of qualitative characters for both female and male goats are presented in Table 2. The result showed that both female and male goat exhibited white, brown, black, grey and cream white coat color type but in varying proportion in either same sex or across two sexes. In all white, brown, black, grey and creamy white coat color type were observed in the sampled goats. The overall (pooled) results showed that proportion of brown, black, white, cream white and grey coat colour were in descending order in the sampled goats. The highest proportion of brown coat colour indicated that farmers prefer this coat colour and have selected these animals favourably. Three coat colour patterns, viz: plain, patchy and spotted, were found in sampled goats. The plain coat colour pattern was dominant with 91.2 % (overall / pooled) occurrence in the sampled goats. The other two coat colour patterns (patch and spotted) were less common.
Quantitative characteristics
Effect of sex
The effect of sex was highly significant (P < 0.01) on body weight and all body measurements. Perusal of least square means (Table 3) showed that body weight and all body measurements in male goats were consistently higher in magnitude than the corresponding values in females. The mean BWT, BL, HG, HW, CW, PW, RH, RL, EL and HL of females were 23.74±0.14 kg, 55.64±0.22cm, 70.07±0.20 cm, 64.03±0.18 cm, 13.68±0.07 cm, 12.47±0.06 cm, 64.23±0.18 cm, 11.90±0.08 cm, 13.45±0.05 cm and 11.19±0.14 cm, respectively. The corresponding values for male counterpart were 26.34±0.21 kg, 3.17±0.033 cm, 59.72±0.34 cm, 74.37±0.31 cm, 68.03±0.05 cm, 14.27±0.12 cm, 68.10±0.12 cm, 12.52±0.13 cm, 13.86±0.08 cm, and 12.99±0.16 cm, respectively.
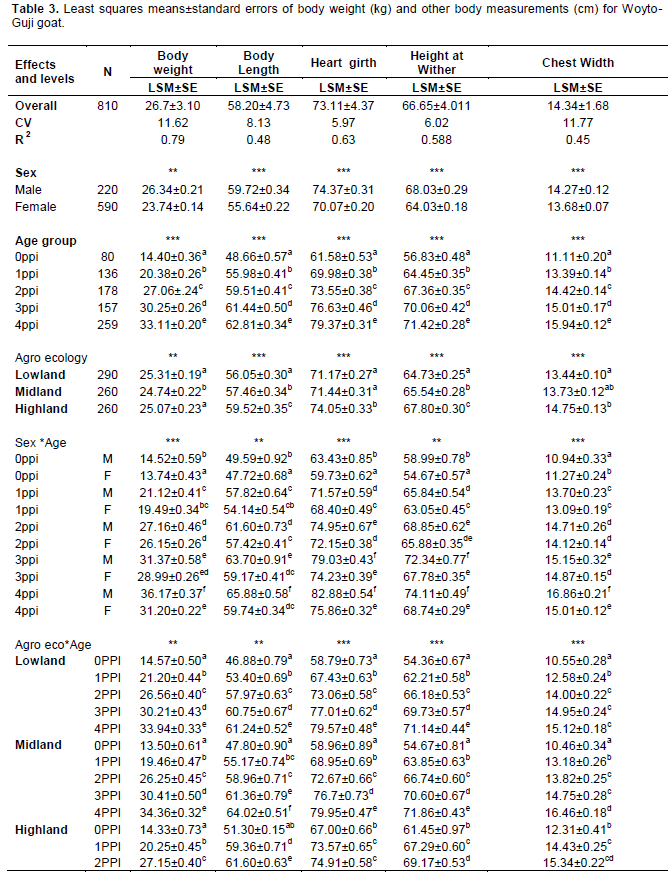
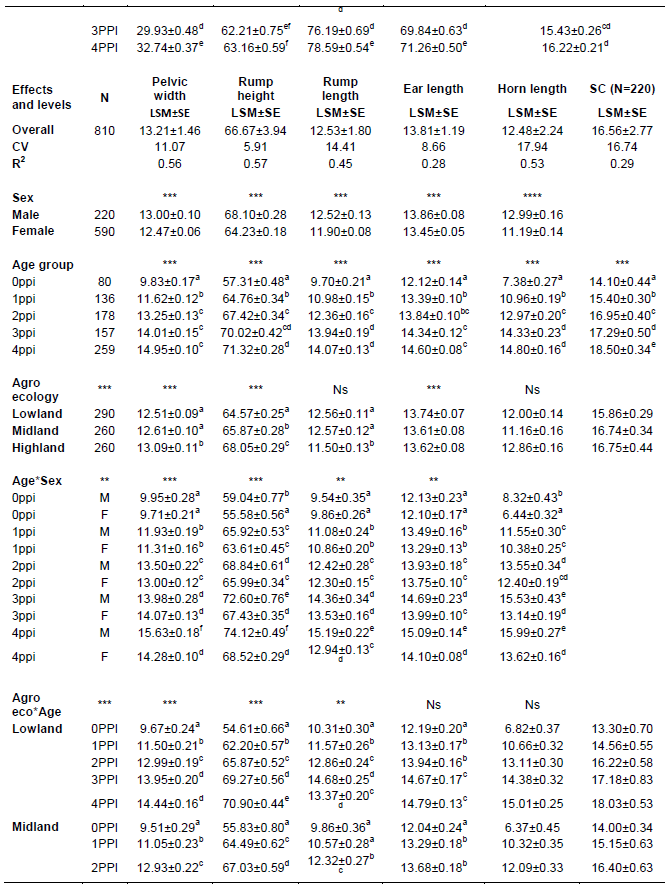
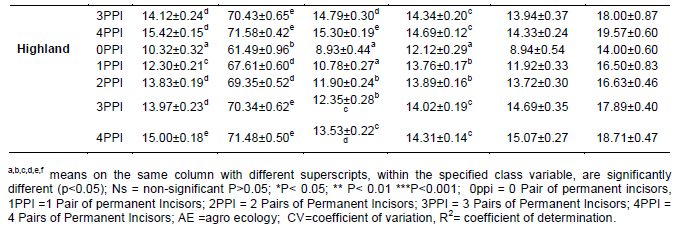
a,b,c,d,e,f means on the same column with different superscripts, within the specified class variable, are significantly different (p<0.05); Ns = non-significant P>0.05; *P< 0.05; ** P< 0.01 ***P<0.001; 0ppi = 0 Pair of permanent incisors, 1PPI =1 Pair of permanent Incisors; 2PPI = 2 Pairs of Permanent Incisors; 3PPI = 3 Pairs of Permanent Incisors; 4PPI = 4 Pairs of Permanent Incisors; AE =agro ecology; CV=coefficient of variation, R2= coefficient of determination.
The effect of sex in favor of males on body weight and body measurements in present study was in agreement with previous results (Semakula et al., 2010, Solomon, 2014, Vargas et al., 2007). The sex related differences might be partly a function of the sex differential hormonal effect on growth. In addition to that, the differentials obtained in the morphological traits of the sexes could be attributed to sexual dimorphism (Semakula, 2010). Peter et al. (2012) reported that most dimorphism developed post-weaning because of faster mass gain by males during the age of 1 to 2 years. They also suggested that males might have a longer season of mass gain each year throughout their lives, while females divert annual resources into reproduction, rather than body mass.
Effect of age groups
The effect of age was highly significant (P < 0.01) on body weight and all other body measurements. Perusal of least square means showed that both body weight and linear body measurements have shown a consistent increase with advancement in age from the youngest age (0PPI) to the oldest age (4PPI) in the present study. These results were in agreement with earlier reports of increase in live body weight and linear body measurements with increase in age of animal in all breeds of goat as (Semakula et al., 2010, Solomon, 2014).
Effect of agro ecology
The effect of Agro ecologies were highly significant (P < 0.01) for all traits, studied, except ear length and scrotal circumference were not significant (P>0.05). Perusal of least square means showed a consistently ascending trend in the measurements from lowland to highland Agro ecologies for BL, HG, HtW, CW, PW and RH. In other traits no such consistent trend was observed. General lower values were observed in most of the linear measurements for lowland Agro ecologies compared to other. This might, to some extent, be explained by environmental factors such as nutrition. In this regard, the reported shortage of grazing areas in the site could be implicated. Grazing lands in the area have been under increased encroachment by the mounting industrial and settlement buildings in line with expansion of the urban core. Owing to the fact that the farming system is dependent on extensive grazing without supplementation, the size and productivity of the grazing land can be taken as the sole component of the environmental factors affecting livestock productivity. The present finding reflected that there were wide variations among the three Agro ecologies which influenced all the quantitative traits studied. The present results were in agreement with earlier study showed that the effects of Agro ecologies was significantly affected on body measurements in indigenous goat breeds (Solomon, 2014; Grum, 2010).
Effect of sex x age groups interaction
The interaction between sex and age groups were either highly significant (P < 0.01) or significant (P < 0.05) on body weight and all body measurements except scrotal circumference which was not studied. The results (Table 3) showed that the magnitude of values of body weight and all other body measurements were consistently higher in males of different age groups than corresponding values for females of various age groups. The pairwise comparison of means showed variable trends in all the traits studied. The present findings were in agreement with earlier studies of (Fajemilehin and Salako, 2008; Dereje, 2011) which reported significant influence of sex and age interaction on body measurements. Hence, this finding should be considered in improvement program to increase meat yield from goat via sex disintegrated improved management.
Effect of agro ecology X age group interaction
The interaction between Agro ecologies and age groups was either highly significant (P < 0.01) or significant (P <0.05) on body weight and all body measurements except horn length and scrotal circumference. These results indicated that effect of Agro ecologies was different in different age groups and thus variation in the Agro ecologies has a strong effect on quantitative traits. There are several advantages of considering this interaction from genetic improvements and conservation perspectives. Advantage of Agro ecologies by age interaction is primarily the active breeding of animal populations for food and agriculture, such that diversity is best utilized in the short term and maintained for the longer term. In addition to that it is useful to conserves both the genetic material and the processes that give rise to the diversity in its production environment with age group.
Correlation between body weight and body measurements
Heart girth had the highest correlation with body weight at various ages and in both sexes compared with other parameters, except in females of zero dentition was not significant correlated as indicated in Table 4. The high correlation between body weight and heart girth, observed in majority of age groups, in present study suggested that heart girth could be used to obtain more reliable prediction estimate of body weight for the population. The present results were also supported by reports of Grum (2010), Dereje (2011), Badi et al. (2002), Slippers et al. (2000) and Halima et al. (2012) where they found that chest girth was best parameter for estimating body weight due to high correlation estimates.
Multiple regression analysis
Perusal of results revealed that heart girth (HG) has been selected across four age groups in female (1PPI, 2PPI, 3PPI and 4PPI), five age groups in male (0PPI, 1PPI, 2PPI, 3PPI and 4PPI) and pooled overall age groups in both sex as presented in Table 5a and b, the first regressor because of its high contribution in terms of R2 values. The regression equation for pooled overall age groups was estimated as Y= (-28.20) + 0.74 X; (where X stands for HG), with R2 value of 0.68 for female and Y = (-39.12) + 0.88 X (where X stands for HG), with R2 value of 0.78 for male goat in the present study. This finding showed that an increase of 1 cm of HG resulted in an increase of 0.74 and 0.78 kg of live weight in female and male goats, respectively.
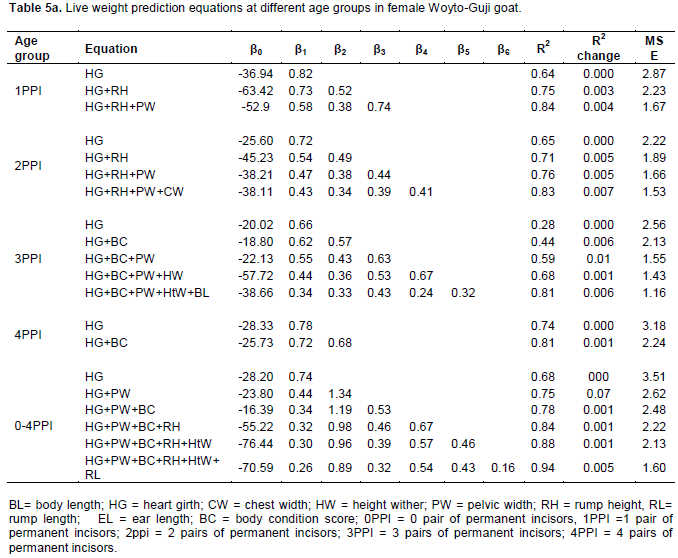
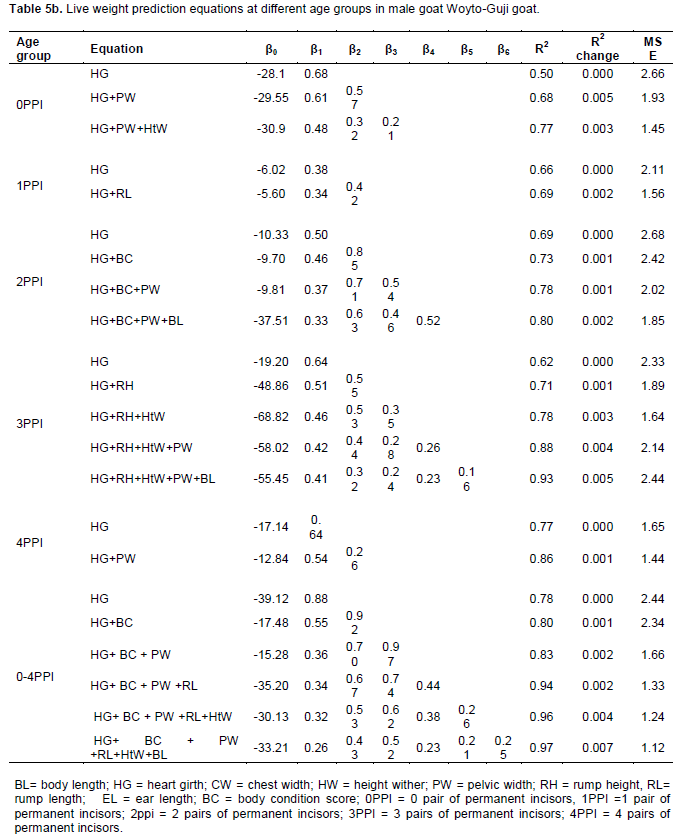
The role of other body measurements’ in predicting live body weight differed in different age groups across the two sexes vis-à-vis their order in these equations. Thus it seems that body measurements other than HG may not possibly be used in general prediction equations. However the parameter estimates in multiple linear regression models showed that subsequent inclusions of other body measurements together with HG (First variable in all equations) kept the R2 values improving although the change had a pattern of diminishing marginal rate. This suggested that body weight could be more accurately predicted by combinations of two or more measurements than heart girth alone. The earlier reports have also shown improvement in R2 values with subsequent addition of more linear measurements (Gul et al., 2005; Fikrte, 2008; Zewdu, 2008). Nevertheless, measurement of traits also has cost implications and it will be impractical to consider many traits under farmer’s conditions. Under such conditions, the most practical prediction accuracy may be obtained through the use of heart girth alone.
CONCLUSION AND RECOMMENDATION
All the body measurements in male goats were consistently higher than females for all variables. The effect of sex, age and agro ecologies was highly significant (P < 0.01) on body weight and majority of body measurements. This should be considered in improvement program to increase production and productivity from goat via sex, Age and Agro ecologies disintegrated improved management. The results of the present investigation could assist farmers and genetic improvement specialists when conducting management, selection and preservation programs for the Woyto-Guji goats. The goats have shown variation across agro ecologies that might be because of various environmental stress, feeding system, prevailing breeding practices, practice of grazing land and management. Therefore, attention should be given for their improvement, conservation, breeding management and for proper utilization to further explore the potential of this genetic material through improving genetic and husbandry management.
The authors have not declared any conflict of interest.
The author would like to thanks Jimma University College of Agricultural and Veterinary Medicine for their financial support.
REFERENCES
|
Alemayehu R (1993). Characterisation (Phenotypic) of Indigenous Goats and Goat Husbandry Practices in East and South-Eastern Ethiopia. An MSc thesis, Alemaya Univer. Agric. Ethiopia. P. 135. |
|
|
|
Aschalew T, Sisay L, Ameha S, Abebe M, Zinash S (2000). National Goat Research Strategies in Ethiopia, pp: 1-5 In: RC. Merkel, A. Girma and A.L. Goetsch, (eds). The opportunities and Challenges of enhancing goat production in East Africa 10-12 November, 2000. Debub University, Awasa, Ethiopia. |
|
|
|
Badi AMI, Fissehaye N, Rattan PJS (2002). Estimation of live body weight in Eritrean goat from heart girth and height at withers. Indian J. Anim. Sci. 72:893-895. |
|
|
|
Bizhan M, Mansour B, Reza S, Majnun S, Hamed A (2010). Genetic Diversity among Three Goat Populations Assessed by Microsatellite DNA Markers in Iran. J. Global Vet. 4(2):118-124. |
|
|
|
CSA (Central Statistical Authority). (2011). Agricultural sample survey 2010/11. Report on livestock and livestock characteristics. Vol. II, Stat. BullAddis Ababa, Ethiopia P. 505. |
|
|
|
Dereje T (2011). Community based characterization of Hararghe high land goats in Darolabu district Western Hararghe, An MSc Thesis presented to the school of Gradute studies of Jimma University. Jimma, Ethiopia. |
|
|
|
Dereje T, Berhanu B, Aynalem H (2013). Morphological characterization of indigenous Haraghe goat breed in their native environment. Am. Eur. J. Scient. Res. 8(2):72-79. |
|
|
|
Fajemilehin SOK, Salako AE (2008). Body measurement characteristics of the West Dwarf (WAD) goat in deciduous forest zone of southwestern Nigeria. Afr. J. Biotechnol. 7(14):2521-2526. |
|
|
|
FARM-Africa (1996). Goat Types of Ethiopia and Eritrea. Physical description and Management systems. Published jointly by FARM-Africa, London, UK and International Livestock Research Institute, Nairobi, Kenya. |
|
|
|
Fikrte F (2008). On-farm characterization of Blackhead Somali sheep breed and its production system in Shinile and Erer districts of Shinile zone. An M.Sc Thesis presented to the school of Graduate Studies of Haramaya University of Agriculture, Dire Dawa, Ethiopia. P. 134. |
|
|
|
Grum G (2010). Community-Based Participatory Characterization of the Short Eared Somali Goat population around Dire Dawa. An MSc Thesis Presented to the School of Graduate Studies of Haramaya University. P. 146. |
|
|
|
Gul S, Gorgulu O, Keskin M, Bicer O, San A (2005). Some Prediction Equations of Live Weight from Different Body measurements in Shami (Damascus) Goats. J. An. Veter. Advan. 4(5):532-534. |
|
|
Halima H, Lababidi S, Rischkowsky B, Baum M, Markos T (2012). Molecular characterization of Ethiopian indigenous goat populations. Trop Anim Health Prod DOI 10.1007/s11250-011-0064-2, 1-8.
Crossref |
|
|
|
IBC (Institute of Biodiversity Conservation) (2004). State of Ethiopia's Farm Animal Genetic Resources country report. A contribution to the first report on the state of the world's animal genetic resources. IBC. Addis Ababa Ethiopia. |
|
|
|
Itty P, Ankers P, Zinsstag J, Trawally S, fister PK (1997). Productivity and profitability of Sheep Production in the Gambia: Implications for Livestock Development in West Africa. Q. J. Int. Agric. 36:153-172. |
|
|
|
LAR (2013). Loma Administrative Annual report. Loma Woreda Administrative Agricultural office annual report. Gessa. Ethiopia. P. 24. |
|
|
|
Mathewos A (2008). Ethnobotany of Spices, Condiments and Medicinal Plants in Loma and Gena Bosa Woredas of Dawro Zone, Southern Ethiopia. A Thesis Submitted to the School of Graduate Studies in Addis Ababa University in Partial Fulfilment of the Degree of Masters in Biology (Dryland Biodiversity). P. 120. |
|
|
|
MOA (Ministry of Agriculture) (2000). Agro ecological Zonation of Ethiopia. Addis Ababa, Ethiopia. |
|
|
Birteeb PT, Peters SO, Yakubu A, Adeleke MA, Ozoje MO (2012). Multivariate characterisation of the phenotypic traits of Djallonke and Sahel sheep in Northern Ghana. Trop. An. Health Prod. 45(1):267-74.
Crossref |
|
|
|
Salako AE (2006). Principal component factor analysis of the morpho-structure of immature uda sheep. Int. J. Morphol. 24(4):571-774. |
|
|
|
SAS (Statistical Analysis System) (2010). Statistical Analysis System Ver 9.2. SAS Institute Inc. Cary. North Carolina, USA. |
|
|
|
Semakula J, Mutetikka D, Kugonza DR, Mpairwe D (2010) Variability in body morphometric measurements and their application in predicting live body weight of Mubende and Small East African goat breeds in Uganda. Middle-East J. Sci. Res. 5:2. |
|
|
Slippers SC, Letty BA, De Villiers JF (2000). Predicting the body weight of Nguni goats. S. Afr. J. An. Sci. 30:127-128.
Crossref |
|
|
|
SPSS Version, 20.0 (2013). Software Package for Social Sciences for Window. |
|
|
|
Solomon A (2014). Design of community based breeding programs for two indigenous goat breeds of Ethiopia. Doctoral Thesis. January 2014 Vienna, Austria. |
|
|
|
Tatiana S (1999). Teeth and Age of the Goat. New York state 4-h meat goat project fact sheet 11, Cornell University, Ithaca, P. 14853. |
|
|
|
Tesfaye AT (2004). Genetic characterization of indigenous goat populations of Ethiopia using Microsatellite DNA markers. PhD Thesis, NDRI, India, P. 215. |
|
|
|
Vargas S, Larbi A, Sanchez M (2007). Analysis of size and conformation of native Creole goat breeds and crossbreds used in smallholder agrosilvopastoral systems in Puebla, Mexico. TroAnim. Health Prod. 39:279–286. |
|
|
|
Workneh A (1992). Preliminary Survey of Indigenous Goat Types and Goat Husbandry Practices in Southern Ethiopia. An M.S.c. Thesis Presented to School of Graduate Studies of Alemaya Univ., of Agriculture, Ethiopia P. 170. |
|
|
|
Workneh A, Rowlands J (2004). Design, execution and analysis of the Livestock breed survey in Oromiya Regional State, Ethiopia. OADB (Oromiya Agricultural Development Bureau), Addis Ababa, Ethiopia, ILRI (International Livestock Research Institute), Nairobi, Kenya. |
|
|
|
Zewdu E (2008). Characterization of Bonga and Horro indigenous Sheep breeds of smallholders for designing Community based breeding strategies In Ethiopia. An MSc Thesis Presented to the School of Graduate Studies of Haramaya University, Haramaya. 143:55-68. |