ABSTRACT
The present study evaluated the anti-nutritional factors of “Bengal” lychee (Litchi chinensis Sonn.), in the fresh pulp, peel and seed, both fresh and processed. The samples were analyzed for the levels of phenolic compounds, nitrate, oxalic acid and inhibitory activities of trypsin, lipase and α-amylase. Drying influenced the activity of all enzyme inhibitors, resulting in a reduction in the inhibitory activity of lipase (0.13 and 0.15 lipase inhibitor units for peel and seed, respectively) and an increase in the inhibitory activities of trypsin (10.14 and 10.66 trypsin inhibitor units for peel and seed, respectively) and α-amylase (1.13 and 1.08 amylase inhibitor units for peel and seed, respectively). With drying, it was possible to observe an increase in the levels of phenolic compounds, the low content of nitrate did not change with drying, while oxalic acid was not detected. The antinutrients evaluated in lychee fractions are present in amounts that do not preclude its use; thus, the use of lychee fractions, fresh or dried, is feasible as nutrient sources and add value to the fruits, since industries can use these residues for developing new products, as well as in food enrichment.
Key words: Phenolic compounds, nitrate, oxalic acid, enzyme inhibitors.
Consumer interest for exotic fruits increases every day because of their nutraceutical value and the correlation between healthy eating and the reduction in the risk of diseases and cancer development (Ferguson and Schlothauer, 2012).
Fruits and vegetables have a large amount of substances capable of providing health benefits by preventing or treating diseases, which are called bioactive compounds. These substances can act in different ways: as antioxidants, activating hepatic detoxification enzymes, inhibiting cholesterol absorption or reducing platelet aggregation. In addition to these compounds, fruits and vegetables may also have anti-nutritional compounds that have deleterious effects on the organism, interfering with digestion and nutrient absorption, or being toxic, depending on the amount in which they are consumed (Pennington, 2002; Suneja et al., 2011; Sreerama et al., 2012; Coimbra and Jorge, 2013). These anti-nutrients such as trypsin inhibitors, lectins, oxalic acid, nitrates and phytates, are present in raw or processed foods (Jain et al., 2009); however, in general, heat processing causes a reduction in the content of some of these compounds, such as protease inhibitors, tannins and lectins (Muzquiz et al., 2006).
In the food industry, fruit processing creates a substantial amount of residues and the recovery of these residues can be an important alternative for sustainable development. In this context, lychee (Litchi chinensis Sonn) is a fruit natural to China, and has been widely cultivated in warm climates around the world (Zhang et al., 2013). It presents a short postharvest life and can be consumed fresh, canned, dehydrated, and processed into juices, fermented beverages, liqueurs, ice creams, etc. Lychee by-products, such as the peel and the seed, are usually discarded by the industry and consumers, and can be used as an alternative source of nutrients, since they do not present anti-nutritional factors in harmful amounts to the body.
Research has shown that lychee by-products have a high energy and nutritional potential, besides anti-inflammatory, anti-hyperlipidemic, anti-hyperglycemic, hepato and cardioprotective activities (Bhoopat et al., 2011; Queiroz et al., 2012; Jiang et al., 2013). Since these by-products have lower storage times, due to water content, drying arises as a tool, as it allows weight reduction, which reduces shipping, packaging and storage costs, besides extending storage time, allowing the application of these farinaceous products as ingredients in the food industry. However, in order to be used, it is necessary to investigate anti-nutritional factors present in lychee fractions and the effect of drying on these factors.
Seeking the discovery of new nutritional sources, as well as the minimization of postharvest losses and the use of agricultural by-products, the fractions peel, pulp and seed of fresh lychee were studied, as well as the peel and the seed dried at 45°C, in order to determine the bioactive compounds with anti-nutritional characteristics.
Raw material and sample preparation
Lychee fruits from Bengal cultivar were picked in December, at a commercial orchard in Nepomuceno, located in the southern region of the state of Minas Gerais (-21°20’S; 45°23’W), Brazil. The lychees were selected according to uniformity of coloration (intense red peel), average size and absence of defects. After the selection of 280 fruits, they were washed, sanitized with 200 μ/L-1 sodium dichloroisocyanurate for 15 m, weighed, divided into 2 batches, each with 7 repetitions with 20 fruits each, and separated into fractions (peel, pulp and seed).
The peel, pulp and seed fractions of the first batch (fresh fruit) were frozen in liquid nitrogen and stored in a freezer (-20°C) until the analysis was carried out. The peel and seed fractions of the second batch (dried) were oven-dried (45°C) until constant weight and stored in an amber flask for approximately 4 days, for the drying of the peel, and 8 days for the seed.
Moisture
The moisture content of lychee peel, pulp and seed was determined by gravimetry, according to the methodology of the Association of Official Analytical Chemists (Horowitz, 2010).
Phenolic compounds
The extraction of phenolic compounds was performed with 50% methanol in reflux for three consecutive times, at 80°C. The extracts were combined, evaporated to 25 ml and subjected to the determination of phenolic compounds, using the Folin-Denis reagent. The results were expressed in tannic acid equivalents (Horowitz, 2010).
Oxalic Acid
The content of oxalic acid was determined by Huang and Tanudjaja (1992), in which the oxalic acid was extracted at 90°C with 6N hydrochloric acid, precipitated and quantified by titration with 0.02 N potassium permanganate.
Nitrates
Nitrates were determined as proposed by Cataldo et al. (1975), by the nitration of salicylic acid under highly acidic conditions dry samples were suspended in deionized water, incubated at 45° C for 1 h, centrifuged at 5,000 x g for 15 m and the supernatant was collected for analysis. The complex formed had its absorbance measured at 410 nm and the results were expressed in mg nitrate 100g-1 dry matter (DM).
Trypsin inhibitory activity
Trypsin inhibitory activity of lychee fraction extracts was determined using the method proposed by Erlanger et al. (1961), using Nα-Benzoyl-DL-arginine-p-nitroanilide hydrochloride (BApNA), prepared in 0.05 mol L-1 TRIS, pH 8.2, as a substrate. The determination of the inhibitory activity was performed from the difference between the activity in the absence (control, without extracts) and in the presence of the extracts. The results were expressed in Trypsin Inibitor Units (TIU) where 1 TIU is equal to 1μmol min-1 per g of DM that fails to be produced due to the presence of the extract.
α -Amilase inhibitory activity
The inhibitory activity of α-amylase in lychee fraction extracts was determined according to Simão et al. (2012). Aqueous extracts of lychee fractions were prepared at a 1:10 (w/v) ratio under horizontal agitation at 4°C, for 30 m. A 50 μL aliquot of the extracts was pre-incubated with 50 μL α-amylase for 20 m, in a water bath at 37°C; soon afterwards, 100 μL of the substratum was added (1% starch solution prepared in a 0.05 mol L-1 Tris buffer, pH 7.0, added with 38 mmol L-1 NaCl and 0.1 mmol L-1 CaCl2), at 4 different times. The reaction was stopped with 200 μL dinitrosalicylic acid (DNS) and the reaction product was measured in a spectrophotometer at 540 nm. The results were expressed in α-Amylase Inibitor Units (AIU), in which 1UIA is equal to 1 μmol min-1 per g of DM that fails to be produced due to the presence of the extract.
Lipase inhibitory activity
Lipase activity was determined according to Souza et al. (2011). A sample of 0.5 g lychee fractions was extracted in 20 ml of 80% ethanol, in an ultrasonic bath, at 4°C, for 30 m. Ethanol was removed from the extract by evaporation and the sample residue was suspended in 5 ml water. A 50 μL aliquot of the extract, at a 1:10 (w/v) ratio, was pre-incubated with 100 μL pancreatic lipase, at 37°C, for 10 m. The reaction was started with the addition of 50 μL of the substrate p-nitrophenyl-palmitate, at 8 mmol L-1, in 0.05 mmol L-1 Tris-HCl, pH 8, containing 0.1% Triton-X100, in a bath at 37°C, and was stopped by transferring the reaction mixture to an ice bath and adding 1000 μL of 0.05 mmol L-1 Tris-HCl buffer. The samples were read in a spectrophotometer at 410nm and lipase activity was expressed in Lipase Inibitor Units (LIU), where 1 LIU is equal to 1μmol min-1 per g of DM that fails to be produced due to the presence of the extract; and inhibition percentage calculated through the difference of the slope of the standard and control curves.
Statistical analysis
The experiment was carried out in a randomized design (CRD), made up of 5 treatments (fresh peel, pulp and seed, and peel and seed dried at 45°C), using 7 repetitions of 20 fruits each. The results were reported as mean ± standard error of mean (SEM), and were submitted to a variance analysis by the SISVAR® statistical software, version 5.1 (Ferreira, 2011), and the averages of the treatments were compared by the Tukey test at 5% probability.
Lychee had an average weight of 18.39 g, of which 27.08% were peels, 50.90% pulp and 22.02% seeds. The moisture found in the peel, pulp and seed was 68.93, 83.91 and 47.11 g 100 g-1, respectively. Phenolic compounds, nitrate and oxalic acid contents verified in lychee fractions are shown in Table 1. There was a significant difference in the levels of phenolic compounds and nitrate in the analyzed fractions. Oxalic acid was not detected in any of the analyzed fractions.
Among the fresh fractions, the seed had the lowest content of phenolic compounds (11.45 mg 100 g-1 dry matter), and no significant difference was found between the content of these compounds in the peel and pulp. The content of phenolic compounds observed in the fractions submitted to drying were significantly higher than those obtained in the fresh ones, especially in the dry peel, which had the highest content of phenolic compounds (71.71 mg 100 g-1).
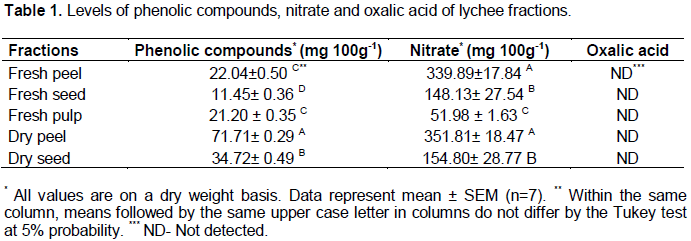
The contents of nitrate ranged from 51.98 to 351.81 mg 100g-1, and the lowest content was observed in the pulp (Table 1). Although processing resulted in significant changes in food composition, drying did not affect the contents of nitrate in the peel and seed, which were very similar to fresh fractions.
Enzymatic inhibitions verified in lychee fraction extracts are listed in Table 2. The fresh peel presented a higher trypsin inhibitory activity than the fresh seed (14.61 and 3.17 TIU, respectively), and inhibition was not found in the pulp. Drying at 45°C influenced trypsin inhibitory activity, increasing it in 10.14 TIU for the peel and in 10.66 TIU for the seed.
The lychee pulp (Table 2) presented a higher α-amylase inhibitory activity (7.23 AIU). The drying process influenced the inhibitory activity of α-amylase; however, there was no significant difference (p< 0.05) between the inhibitory activity in the peel and in the dry seed (1.13 and 1.08 AIU, respectively).
All of the ethanolic extracts of lychee fractions presented inhibitory activity on pancreatic lipase. The pulp presented the highest lipase inhibition percentage (67%), and although all of the extracts had the same concentration, the result expressed in inhibition percentage is relative to extract concentration and, as such, the results were converted to LIU.
Therefore, as occurred in the inhibition percentage, the fresh pulp presented the most expressive inhibitory activity (0.75 LIU), and differences were not observed (p< 0.05) between the inhibitory activities of the fresh peel and the seed.
Drying negatively influenced lipase inhibitory activity, causing a reduction of 0.13 LIU for the peel and 0.15 LIU for the seed, a result that can be observed by the decreases in the inhibition percentage and the inhibitory activity of the dry fractions in relation to the fresh fractions (Table 2).
Dehydration modifies the nutritional value, the physical and structural properties of fruits and vegetables and, even when this occurs in mild temperatures, there is a destruction of cell wall polymers (Ebun and Santosh, 2011; Harbourne et al., 2009; Kim et al., 2006). Therefore, an increase in the contents of phenolic compounds with drying suggests that heating caused a release of phenolic compounds, possibly bound to cell wall polysaccharides that were not extracted, and consequently, quantified in fresh lychee fractions.
Among phenolic compounds, tannins are considered anti-nutrients since, in diets for humans and species of monogastric animals, they can reduce the digestibility of proteins, carbohydrates and minerals; decrease the activity of digestive enzymes, besides causing damage to the lining of the digestive system or having systemic toxic effects (Benevides et al., 2011).
The maximum phenolic compound intake suggested for humans is approximately 1 g day-1 (Scalbert et al., 2005). It is therefore likely that the use of lychee fractions, fresh or dried, is not sufficient to overcome this daily limit. Although processing causes important modifications in food composition, lychee processing did not cause significant changes in nitrate contents. Drying at 45° C is not capable of degrading nitrates present in lychee fractions, and the highest nitrate levels were observed in the peel, both fresh and dry.
The acceptable nitrate daily intake is 5 mg kg-1 body weight (WHO, 2003). The excessive consumption of this compound can cause cyanosis by the formation of metmyoglobin, and neoplasia from the formation of N-nitroso compounds (Faquin and Andrade, 2004). Taking the example of a person weighing 70 kg, they could ingest 350 mg nitrate; thus, these values will only be achieved if the consumption of dried peel fraction with the highest nitrate content, is close to 100 g day-1, a consumption considered relatively high, which reduces the risk of damage caused by high nitrate doses be acquired with the use of dry lychee peel.
A reduction in the content of nitrate was observed in cruciferous vegetables cooked in boiling water and the loss of nitrate is due to the loosening of the plant tissue, with consequent mass increase, as a result of water absorption, thus resulting in its dilution. Nitrate loss was also found in fruits and vegetables submitted to boiling; however, nitrate values remained relatively constant during baking and there was a 2- to 3-fold increase in nitrate content after frying products in soy bean oil (Chetty and Prasad, 2009), which did not occur with dry lychee fractions.
The content of nitrate found in all lychee fractions was lower than that found by Kaminishi and Kita (2006) for spinach, whose average content of nitrate ranged from 3.79 to 4.33 g kg-1 fresh matter (FM), which do not preclude the use of lychee fractions, enabling individual use.
Oxalic acid was not detected in any of the lychee fractions, which represents a favorable result, since oxalate, although often found in plants, cannot be metabolized by humans, and is excreted in the urine. It is estimated that about 75% of kidney stones are comprised of calcium oxalate, and hyperoxaluria is a major risk factor for the disease. Thus, the restriction in oxalate intake in the diet has been suggested as a treatment to prevent recurrent nephrolithiasis in some patients (Benevides et al., 2011).
The effect of processing on the trypsin inhibitory activity of dried fractions is similar to that reported by Naves et al. (2010), who observed a lower activity of these inhibitors in the flour of raw pumpkin seeds, than in those steamed and water-boiled, although the process carried out by that author is different from that performed in lychee peel and seed.
Two of the main types of trypsin inhibitors, Kunitz and Bowman-Birk, are proteic and their thermal stability depends on molecular weight and on the degree of stabilization of the active conformation by disulfide bonds. However, although proteic, the use of moderate temperatures, as used in this study, may not be sufficient to denature such inhibitors, keeping their inhibitory activity. The inactivation of the trypsin inhibitor was only possible combining the treatment under pressure with high temperatures (90% inactivation with the treatment, in less than 2 m, at temperatures between 77 and 90°C and pressures between 750 and 525 MPa) (Vem et al., 2005). This explains the fact that drying at 45°C was not enough to minimize trypsin inhibitor activity of dry lychee fractions.
In spite of the increase due to the drying process, the trypsin inhibitory activity found in all fractions was low in relation to other foods that also present these inhibitors, such as pumpkin seed, white beans, lentil, peanut, chickpea (Naves et al., 2010; Pereira et al., 2010; Pedrosa et al., 2012), and soy, whose values range from 37.73 to 51.68 TIU mg -1 DM (Vem et al., 2005). Many plant families possess inhibitors distributed in various organs, and their expression is constitutive (reproductive organs, reserve organs and vegetative tissue) or induced (response to herbivory, pathogens, mechanical injury and stress) (Chen et al., 2004). The highest trypsin and α-amylase inhibitory activities found in dry lychee fractions are due to the response of the vegetable to the damage due to the process, at the initial drying moments. Although an increase in α-amylase inhibitory activity has occurred with drying, amylase inhibitory activity in the dry fractions was lower than that in the fresh lychee pulp and lower than that found in white bean flour (66.89 AIU) (Pereira et al., 2010).
The highest inhibitory activities of trypsin and α-amylase verified in the dry fractions are probably due to a plant response to the damage caused by the drying process in its early stages (Chen et al. 2004). The reduction in the activity of lipase inhibitors, 0.13 IWU in the peel and 0.15 IWU in the seed (Table 2), suggests that this inhibitor is sensitive to heat treatments. The in vitro enzymatic activity can be influenced by the presence of phenolic compounds of fruits and vegetables that, depending on their structure, can react with proteins and alter various properties of these biopolymers, such as their molecular weight, in vitro digestibility and solubility, showing a nonspecific inhibition (Rohn et al., 2002; Birari and Bhutan, 2007). Lychee pericarp, pulp and seed contain a great amount of polyphenolic compounds, such as condensed tannins, gallic acid, epicatechin, procyanidin A2, anthrocyanin, quercetin 3-rutinoside (rutin), quercetin glucoside and others (Jiang et al., 2013; Zhang et al., 2013), which may exhibit anti-amylase activity, but the distribution of these compounds in lychee fractions varies.
The suppressive effects of this experiment suggest that lychee fractions, especially the pulp, are able to inhibit α-amylase and lipase. Thus, the inhibitory activity of α-amylase found in this study may be caused by the phenolic compounds present in the lychee fraction extract, and additionally, it is possible that protein–phenolic and/or phenolic–phenolic synergies may be involved in the food extract enzyme-inhibition mechanism. Among the compounds that may exhibit potential effects to prevent obesity, polyphenol stands out for inhibiting enzymes related to lipid metabolism, including pancreatic lipase, lipoprotein lipase, and glycerophosphate dehydrogenase. Suppressive effects of polyphenols on lipase activity are due to their affinity for protein and lipase aggregation (Birari and Bhutan, 2007), which can cause their precipitation.
Research with the objective to determine the inhibition of pancreatic lipase in lychee flower reported that aqueous extracts of lychee flower rich in polyphenols show anti-obesity and anti-inflammatory potentials. The results showed that lychee flower-water extracts have suppressive effects against pancreatic lipase activity, with a 45% inhibition in in vitro pancreatic lipase activity and decreased epididymal adipose tissue sizes, as well as decreased serum and in vivo liver lipid contents (Wu et al., 2013).
The percentage of inhibition, in vitro, against pancreatic lipase activities observed in this study is higher than that reported by Wu et al. (2013), and this is probably due to the solvent used for the preparation of the extract and the lychee fraction analyzed.
Suppressive results of this experiment suggest that lychee fractions, particularly the pulp, are capable of inhibiting lipase and α-amylase, and may be helpful in the treatment of obesity caused by the elevation of fat and carbohydrate levels in the diet, although in vivo anti-obesity effects of lychee fractions were not investigated in this study. It should be noted, however, that, despite the high inhibition observed in the pulp, this fraction is rich in carbohydrates, which reinforces the need for future studies on the in vivo inhibitory potential of lychee fractions.
The results of this study show that lychee fractions have anti-nutrients such as phenolic compounds, nitrate and trypsin inhibitors, α-amylase and lipase, in amounts that do not preclude the use of these fractions, fresh or subjected to drying at 45° C, as nutrient sources. Therefore, it is possible to add value to lychee, since industries could use residues (peel and seeds) for commercial formulations and food enrichment.
The authors have not declared any conflict of interest.
REFERENCES
|
Benevides CMJ, Souza MV, Souza RDB, Lopes MV (2011). Fatores antinutricionais em alimentos: revisão. Segur. Aliment. Nutr. 18(2):67-79. |
|
|
Bhoopat L, Srichairatanakool S, Kanjanapothic D, Taesotikul T, Thananchai H, Bhoopat T (2011). Hepatoprotective effects of lychee (Litchi chinensis Sonn.): a combination of antioxidant and anti-apoptotic activities. J. Ethnopharmacol. 136(1): 55-66.
CrossRef |
|
|
Birari RB, Bhutan KK (2007). Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 12(19-20):879-889.
CrossRef |
|
|
Cataldo DA, Hardoon M, Schrader LE, Youngs VL (1975). Rapid calorimetric determination of nitrates in plants tissue by nitration of salicytic acid. Soil Plants Analysts. 6(1):71-80.
CrossRef |
|
|
Chen TE, Huang DJ, Lin YH (2004). Isolation and characterization of a serine proteinase from the storage roots of sweet potato (Ipomea batatas [L.] Lam.). Plant Sci. 166(4):1019-1026.
http://dx.doi.org/10.1016/j.plantsci.2003.12.018 |
|
|
Chetty A, Prasad S (2009). Flow injection analysis of nitrate-N determination in root vegetables: study of the effects of cooking. Food Chem. 116(2):561-566.
CrossRef |
|
|
|
Coimbra MC, Jorge NB (2013). Phenolic compounds, carotenoids, tocopherols and fatty acids present in oils extracted from palm fruits. B. CEPPA 31(2):309-320. |
|
|
|
Ebun O, Santosh K (2011). Effect of domestic cooking on the polyphenolic content and antioxidant capacity of plantain (Musa paradisiaca). World J. Dairy Food Sci. 6(2):189-194. |
|
|
Erlanger BF, Kokowsky N, Cohen W (1961). The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 95(2):271-278.
CrossRef |
|
|
|
Faquin V, Andrade AT (2004). Acúmulo de nitrato em hortaliças e saúde humana. Lavras: UFLA/FAEPE, P. 88. |
|
|
Ferguson LR, Schlothauer RC (2012). The potential role of nutritional genomics tools in validating high health foods for cancer control: Broccoli as example. Mol. Nutr. Food Res. 56(1):126-146.
CrossRef |
|
|
|
Ferreira DF (2011). Sisvar: a computer statistical analysis system. Ciênc. Agrotec. 35(6):1039-1042. |
|
|
|
Harbourne N, Marete E, Jacquier JC, O'riordan D (2009). Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). Food Sci. Technol. 42(9):1468-1473. |
|
|
|
Horowitz W (2010). Official methods of analysis of the Association of Official Analytical Chemists. 18th ed., 3rd rev. Gaithersburg, Maryland: AOAC. |
|
|
Huang AS, Tanudjaja LS (1992). Application of anion-exchange hight-performace liquid chromatography in determining oxalates in taro (Colocasia esculenta) corms. J. Agric. Food Chem. 40(11):2123-2126.
CrossRef |
|
|
|
Jain AK, Kumar S, Panwar JD (2009). Antinutritional factors and their detoxification in pulses - A review. Agric. Res. Rev. 30(1):64-70. |
|
|
Jiang G, Lin S, Wen L, Jiang Y, Zhao M, Chen F (2013). Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 136(2):563-568.
CrossRef |
|
|
|
Kaminishi A, Kita N (2006). Seasonal change of nitrate and oxalate concentration in relation to the growth rate spinach cultivars. HortScience. 4(7):1589-1595. |
|
|
Kim SY, Jeong SM, Park WP, Nam KC, Ahn DU, Lee SC (2006). Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 97(3):472-479.
CrossRef |
|
|
|
Muzquiz M, Pedrosa MM, Varela EAJ, Guillamon E, Goyoaga C, Cuadrado C, Burbano C (2006). Factores no-nutritivos en Fuentes Proteicas de Origen Vegetal. Su Implicación en Nutrición y Salud. Braz. J. Food Technol. III JIPCA: 87-98. |
|
|
Naves LP, Correa AD, Santos CD, Abreu CMP (2010). Componentes antinutricionais e digestibilidade proteica em sementes de abóbora (Cucurbita maxima) submetidas a diferentes processamentos. Ciênc. Tecnol. Aliment. 30(1):180-184.
CrossRef |
|
|
Pedrosa MM, Cuadrado C, Burbano C, Allaf K, Haddad J, Gelencsér E, Takács K, Guillamón E, Muzquiz M (2012). Effect of instant controlled pressure drop on the oligosaccharides, inositol phosphates, trypsin inhibitors and lectins contents of different legumes. Food Chem. 131(3):862–868.
CrossRef |
|
|
Pennington JAT (2002). Food Composition Databases for Bioactive Food Components. J. Food Compost. Anal. 15(4):419-434.
CrossRef |
|
|
|
Pereira LLS, Santos CD, Pereira CA, Marques TR, Sátiro LC (2010). Precipitação do inibidor de α-amilase de feijão branco: avaliação dos métodos. Alim. Nutr. 21(1):15-20. |
|
|
Queiroz ER, Abreu CMP, Oliveira KS (2012). Constituintes químicos das frações de lichia in natura e submetidas à secagem: potencial nutricional dos subprodutos. Rev. Bras. Frutic. 34(4):1174-1179.
CrossRef |
|
|
Rohn S, Rawel HM, Kroll J (2002). Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 50(12):3566-3571.
CrossRef |
|
|
Scalbert A, Johnson IT, Saltmarsh M (2005). Polyphenols: antioxidants and beyond. Am. J. Clin. Nutri. 81(1):215S-217S.
PMid:15640483 |
|
|
|
Simão AA, Corrêa AD, Chagas PMB (2012). Inhibition of digestive enzymes by medicinal plant aqueous extracts used to aid the treatment of obesity. J. Med. Plant Res. 6(47):5826-5830. |
|
|
Souza SP, Pereira LLS, Souza AA, Santos CD (2011). Inhibition of pancreatic lipase by extracts of Baccharis trimera: evaluation of antinutrients and effect on glycosidases. Rev. Bras. Farmacogn. 21(3):450-455.
CrossRef |
|
|
Sreerama YN, Sashikala VB, Pratape VM, Singh V (2012). Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality. Food Chem. 131(2):462–468.
CrossRef |
|
|
Suneja Y, Kaur S, Gupta AK, Kaur N (2011). Levels of nutritional constituents and antinutritional factors in black gram (Vigna mungo L. Hepper). Food Res. Int. 44(2):621-628.
CrossRef |
|
|
Vem CVD, Matser AM, Berg RWVD (2005). Inactivation of soybean trypsin inhibitors and lipoxygenase by high-pressure processing. J. Agr. Food Chem. 53(4):1087-1092.
CrossRef |
|
|
|
World HealtH Organization (2003). WHO Food additives series No 50. Safety evaluation of certain food additives. Fifty-ninth report of the joint FAO/WHO Committee on Food Additives. Geneva. |
|
|
Wu YHS, Chiu CH, Yang DJ, Lin YL, Tseng JK, Chen YC (2013). Inhibitory effects of litchi (Litchi chinensis Sonn.) flower-water extracts on lipase activity and diet-induced obesity. J. Funct. Foods 5(2):923-929.
CrossRef |
|
|
Zhang R, Zeng Q, Deng Y, Zhang M, Wei Z, Zhang Y (2013). Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 136(3-4):1169-1176.
CrossRef |