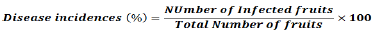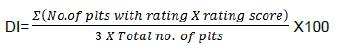ABSTRACT
Tomato (Solanum lycopersicum L.) is a species of the family Solanaceae. It is herbaceous, annual to perennial, prostrate and sexually propagated plant with bisexual flower. Tomatoes are attacked by many kinds of plant pathogens such as fungi, bacteria, nematodes, viruses and viroid. Among bacterial diseases, bacterial soft rot devastates many important crops of the family Solanaceae particularly potato, eggplant and tomato, causing a huge decrease in yield and a greater loss in produce than any bacterial disease known. Yield losses due to post-harvest diseases of fruits and vegetables range from 20 to 30% but losses due to soft rot bacteria may reach up to 100% under insufficient conditions of storage facility, this have huge impacts on famers and vendors. In vitro efficacy of certain botanicals against bacterial soft rot of tomato were tested in the months of February to March, 2015 in the Department of Plant Pathology and Department of Biochemistry, Sam Higginbottom Institute of Agriculture, Technology and Sciences (Deemed University)– Allahabad, UP, India. Eight botanicals were screened in vitro, out of these, four were selected based on their performances and evaluated against the bacterial soft rot of storage tomato at 2, 4, and 8 days after inoculation. Maximum zone of inhibition was obtained with treated Control (T0b=17 mm), followed by Turmeric 30% (T4=12.4 mm), Turmeric 20% (T3=11 mm), then Neem 30% (T6) while the least zone of inhibition was recorded with untreated Control/water (T0a=0.4 mm) followed by Lemon 30% (T12=1 mm). Turmeric 30% (T4) proved to be best botanical under screening followed by Turmeric 20% (T3=11mm). In case of mean disease intensity at eight days after inoculation on storage tomato, highest mean value was recorded in Ginger 30% (T2=46.2) followed by Neem 20% (T5=44.2) and lowest value in Streptomycin (T0b=27), followed by Turmeric 20% (T3=27.6) then Turmeric 30% (T4=27.8). Among the botanicals, the lowest disease intensity was with T3=27.6 followed by T4= 27.8.
Key words: Tomato, Pectobacterium carotovora subsp carotovora, botanicals, efficacy.
Tomato (Solanum lycopersicum L.) belongs to the family Solanaceae (Taylor, 1986; Rashid and Singh, 2000). It is herbaceous, annual to perennial, prostrate and sexually propagated plant with bisexual flower. It is typically day neutral plant and self-pollinated vegetable crop. Scientific information indicates that the cultivated tomato originated in a wild form in the Peru-Ecuado-Bolivia area of the Andes, that is, South America (Vavilov, 1951 and Rick, 1969).
Tomatoes are attacked by many kinds of plant pathogens such as fungi, insects, nematodes, bacteria, viruses and viroid. Among bacterial diseases of tomato, bacterial soft rot devastates this important crop, causing a huge decrease in yield and a greater loss in produce than any bacterial disease known (Akbar et al., 2014). The disease is associated with infection by Pectobacterium species, formally known as Erwinia sp. (Czajkowski et al., 2011) such as Pectobacterium chrysanthemi (Pc), Pectobacterium carotovora subsp. carotovora (P. carotovora subsp. carotovora), and Pectobacterium carotovora subsp. atroseptica (Pca). The latter is also the causal agent of blackleg of potato (Perombelon et al., 1980). Pectobacterium species secrete different degenerative enzymes, including pectate lyases, pectin lyases, polygalacturonases, cellulases, proteases and phospholipases which can depolarize the plant cell wall and macerate tuber parenchymatous tissues (Kotoujansky, 1987).
P. carotovora subsp. carotovora is economically important because of its ability to cause severe soft rot on tomatoes (Perombelon and Kelman, 1980; Akbar et al., 2014). They cause wilting of whole plant, water soaking areas on stem and fruits, browning of vascular tissue and fruits, discoloration of fruits, hollowing of pith and soft rotting of stem and fruits. P. carotovora subsp. carotovora infects a much broader host range including many vegetables, for example, potato and tomato (Perombelon and Kelman, 1980; Bell et al., 2004).
In India, P. carotovora subsp. carotovora is identified as the major soft rot causing bacterium (MCC-Pune, Catalogue 2014). Although control of blackleg and bacterial soft rot with antibiotics have showed to be promising, large scale field studies are no longer encouraged because of the risks of introducing resistance to bacterial pathogens of man and animals. Chemical treatment also have the problem of reaching the pathogen which are well protected in vascular system, lenticels etc. and even systemic bactericide failed when applied postharvest, as there is no vascular activity in harvested fruit or tuber (Czajkowski et al., 2011).
The problems caused by synthetic pesticides and their residues have increased the need for the search of effective biodegradable pesticides with greater selectivity (Al-Samarrai et al., 2012; Slusarenko et al., 2008). The alternative strategies are focused on pesticides of plant origin, which are often effective against a limited number of specific target species, are biodegradable into non-toxic products and suitable for use in integrated pest management programs (Al-Samarrai et al., 2012).
Plant products effectively meet this criterion and have enormous potentials to influence modern agrochemical research. The use of botanicals is gaining popularity because they have been found to be non-toxic, more systemic with little mammalian toxicity (Bankole, 1996). It degrades more rapidly than most chemicals pesticides, and therefore are considered to be eco-friendly and less likely to kill beneficial pests than synthetic pesticides with longer environmental retention.
A-Source of materials used
The bacterial culture MMC-2112 (T) used in the experiment was procured from Microbial Culture Collection Centre-Pune (ncc, 2015), National Centre for Cell Science, Maharashtra State, India.
B-Preparation of plant extracts (botanicals)
Aqueous extracts of easily available plants in Allahabad such as the ones listed below were prepared according to a method described by Obongoya et al. (2010), revised by Paradza et al. (2012) with minor modifications. For the experiment at two concentrations (20% and 30%) for each treatment were as follows:
T1 -Ginger (Zingiber officinale) 20%, T2 -Ginger (Zingiber officinale) 30%, T3-Turmeric (Curcuma longa) 20%, T4-Turmeric (Curcuma longa) 30%, T5-Neem seed (Azadirachta indica) 20%, T6-Neem seed (Azadirachta indica) 30%, T7-Coriander (Coriandrum sativum L.) 20%, T8-Coriander (Coriandrum sativum L.) 30%, T9-Garlic (Allium sativum L.) 20%, T10-Garlic (Allium sativum L.) 30%, T11-Lemon peel (Citrus aurantifolia) 20%, T12-Lemon peel (Citrus aurantifolia) 30%, T13-Black Cumin (Nigella sativa L.) 20%, T14-Black Cumin (Nigella sativa L.) 30%, T15 -Chilli (Capsicum annuum) 20%, T16 -Chilli (Capsicum annuum) 30%, T0a-Sterile distilled water (Untreated Control), T0b-Streptomycin sulphate (Treated control).
The plant materials were first oven dried (except black cumin, turmeric and neem seeds) and grinded into powder, using electric grinder (Mixer Grinder). Dried plant tissues (20 g/100ml and 30g/100ml) were measured and soaked for 24 h in distilled water. Then suspension of each plant extract was filtered using 4 layers muslin cloth, 2 times. Discs of 12.7 mm were soaked in these extracts for 24 h and used as botanical treatment on the bacterial lawns under in vitro screening. While for streptomycin sulphate, only 1 g of powder was used in 100 ml sterile distilled water after which discs were soaked and used as earlier described (positive control). In case of sterile distilled water (negative control), discs were just soaked and used as earlier mentioned.
C-In vitro screening of botanicals against the Pectobacterium carotovora sub sp. carotovora
In vitro screening of botanicals and its optimum concentration was carried out using bacterial zone of inhibition and disc diffusion method (Akbar et al., 2014) with little modification. A young culture of the bacterium (Pectobacterium carotovora subsp. carotovora) 24 to 48 h old was used for the preparation of bacterial lawn. Bacterial culture lawns were prepared by spreading the bacterial culture 107cfu/ml on the growth medium using sterile spreader. Each treatment (plant extracts and checks) was replicated five times and this was applied to the two concentrations. Each of the soaked disc was placed at the centre of a bacterial lawn. The inoculated plates were incubated at room temperature for 24 to 72 h, and the procedure earlier mentioned remained the same for all the extracts and checks. After incubation, data were taken as inhibition zones (mm) around the discs as effects of botanicals against the bacterium (bacterial lawn), promising botanicals were chosen for further experiment on storage tomato fruit.
D-Efficacy of selected botanicals against bacterial soft rot of tomato fruits
Method described by Rahman et al. (2012) was followed with little modifications. Based on the encouraging results of the bioassay as inhibition efficiency and efficacy, four botanical extracts with promising results were chosen to evaluate their efficacy against bacterial soft rot of tomato fruits.
The extracts were prepared as described earlier under screening. Each tomato fruit was drilled with 14 mm cork borer, then treated the cut section with the plant extract for 30 min. Inoculum of the soft rot bacterium was prepared at concentration of 107cfu/ml and 12.7 mm paper discs soaked for 30 min. The plant extract treated tomatoes were inoculated according to Opara et al. (2013) with some modifications where paper discs soaked in the inoculum suspensions107cfu/ml where a disc introduced per fruit’s section, and then top tissues were replaced as cap. Inoculated tomatoes were then incubated in polyethene bags at room temperature along with controls. Visual observations were made at 4 and 8 days after inoculation and data on diameter of soft rotting site (mm) due to treatments were evaluated.
The number of fruits showing symptoms of the diseases in each treatment was counted and the percentage of disease incidence was computed using the following formula:
Disease intensity assessment was carried out using a scale of 1 to 3 (Subrahmanyam et al., 1995; Saleem et al., 2011). Three fruits were selected at random, observed and scored. Based on the extent of observed disease damage on each, a scale number was assigned as follows:
1. “o” no visible symptoms (protected)
2. “1” a few minute lesions, approximately 10% of the total fruit surface (TFA) is rotted (moderately protected)
3. “2” approximately 50% TFA is rotted (unprotected)
4. “3” most fruits surface display symptoms, at least 75% of the TFA is rotted (severely unprotected).
Disease intensity was calculated as per cent using the following formula:
Where;
Σ = Summation symbol
DI = Disease intensity
3 = Highest disease rating score
Plts = plants/fruits
No. = Number/sample size
E-Statistical analysis
The experiment was laid out in completely randomised design (CRD) and WASP-SOFTWARE of Web Agri. Stat. Package from ICAR Research Complex for Goa, India was used to analyse the data.
A-In vitro screening of botanicals against Pectobacterium carotovora sub sp. carotovora
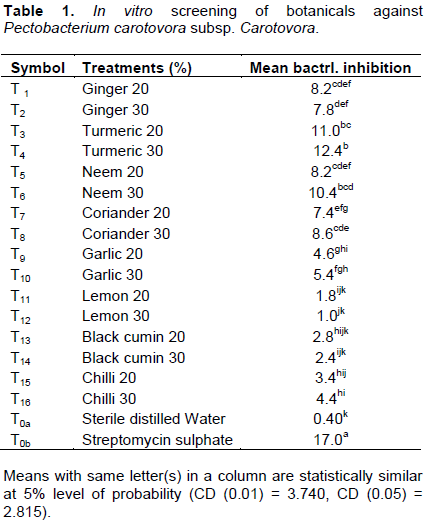
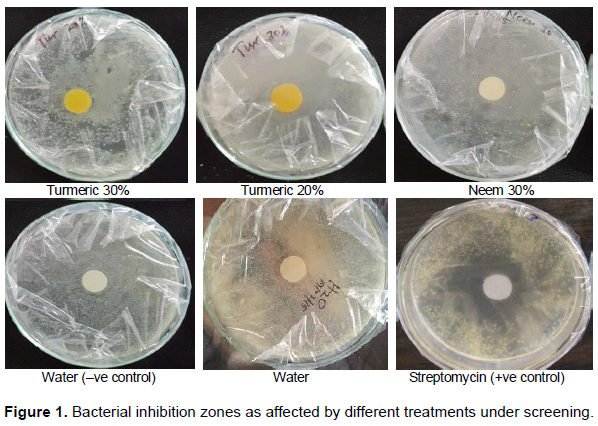
In the in vitro screening of botanicals using disc zone of inhibition, it was observed that growth of P. carotovora sub sp. carotovora was inhibited by most of the tested botanicals when compared to untreated control. Maximum zone of inhibition was obtained with treated control (T0b=17 mm), followed by turmeric 30% (T4=12.4 mm), then turmeric 20% (T3=11 mm), while the least zone of inhibition was recorded with untreated control/water (T0a=0.4 mm) followed by lemon 30% (T12=1 mm) (Table 1 and Figure 1).
B- Efficacy of selected botanicals against bacterial soft rot of tomato during storage
Four botanicals were selected based on their performance as inhibition efficiency and efficacy under screening and evaluated against the bacterial soft rot of tomato under storage condition. Disease incidence/infection (%) was determined according to Rahman et al. (2012).
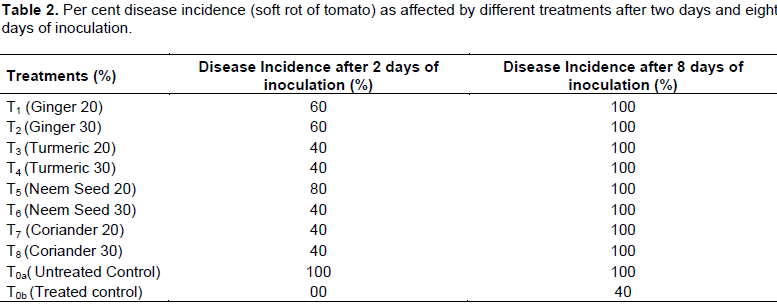
Among the botanicals evaluated turmeric 20% (T3), turmeric 30% (T4), neem 30% (T6), coriander 20% (T7), and coriander 30% (T8) had the lowest disease incidence of 40% at two days after inoculation compared to rest of botanicals. The least among all the treatments was found to be treated control (T0b) with 0% (Table 2).
But incidences of the disease were observed in all the eighteen treatments (that is, including both controls) at eight days after inoculation where incidence of the soft rot disease was found to be 100% in all the treated fruits plus untreated control while in treated control it was found to be only 40% as depicted in Table 2.
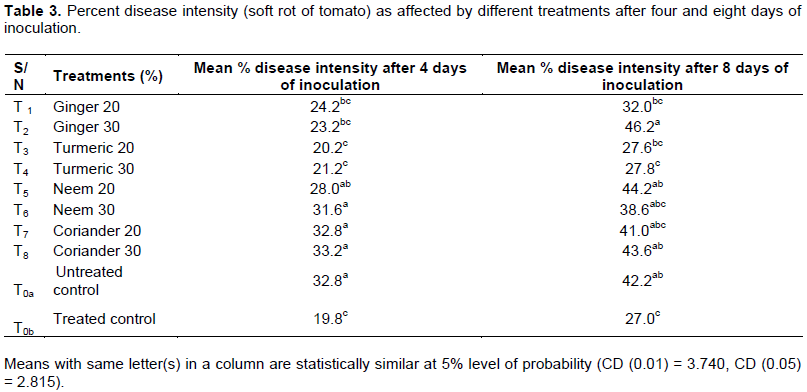
Disease intensity was measured according to Nauvov (1924), Ahmed (1976) and Pangtey (1979). The highest disease intensity at four days after inoculation was recorded in coriander 30% (T8=33.2) which means the disease aggravates early there while lowest appeared in Turmeric 20% (T3=20.2) which is next to treated control/streptomycin (T0b=19.8), (Table 3).
In vitro screening of botanicals against Pectobacterium carotovora sub sp. carotovora
Turmeric 30% (T4=12.4 mm) was found to effectively inhibit the bacterial growth as its second to treated control (T0b=17 mm), and this value agrees with the findings of Akbar et al. (2014), who reported maximum zone of bacterial growth inhibition by turmeric (13.33 mm). Apisariyakul (1995) reported turmeric as potential antimicrobial, antioxidant, antiprotozoal and anti-allergic.
The active ingredient(s) in turmeric needs to be elucidated. The effect of neem (Azadirachta indica) as observed is in agreement with the findings of Opara et al. (2013) and Bhardwaj and Laura (2008), but not in accordance with Paradza et al. (2012).
The microbial activity shown by turmeric may be due to the action of its volatile oil constituent curcumin which has enolizable β-diketo group as chelating ligand (Rachana and Venugopalan, 2014) while Slusarenko et al. (2008) reported neem to have active substance Azadirachtin which is under subclass of compound limonoids, class triterpenes and is active against a wide range of microbes and/or pests with up to 90% efficacy in most cases (Akbar et al., 2014; Koul and Walia, 2009). Neem is reported to have fungicidal activity (Bankole, 1996; Govindachari et al., 1998) and bactericidal activity (Mahfuzul-Haque et al., 2007), while ginger (Zingiber officinale) was reported to have bactericidal effect on Erwinia sp due to its volatile essential oil (Opara et al., 2013).
Efficacy of selected botanicals against bacterial soft rot of tomato during storage
T0b been synthetic antibiotic appeared to be highest as control agent, while the highest incidence was recorded with untreated control (T0a =100%) (Table 2). Disease incidence was common throughout the treatments at 8 days after inoculation; this could be due to development of resistance in some of the inoculum or other microbial complex development (Nauvov, 1924; Pangtey, 1979). It might as well be due to resurfacing and proliferation of the resistant colonies of the pathogen after long period of inhibition. The slight difference in between this work mean values and the previous researchers’ own might be due to difference in the inoculum concentration or atmospheric condition.
The antimicrobial activity shown by turmeric may be due to its chelating action as earlier stated (Rachana and Venugopalan, 2014) but coriander also proved to have antimicrobial activity which may be attributed to its essential oil, known to exhibited bactericidal activity against most gram negative and gram positive bacteria (Silva et al., 2011). Its mode of action is reported to be by membrane damage (Silva et al., 2011).
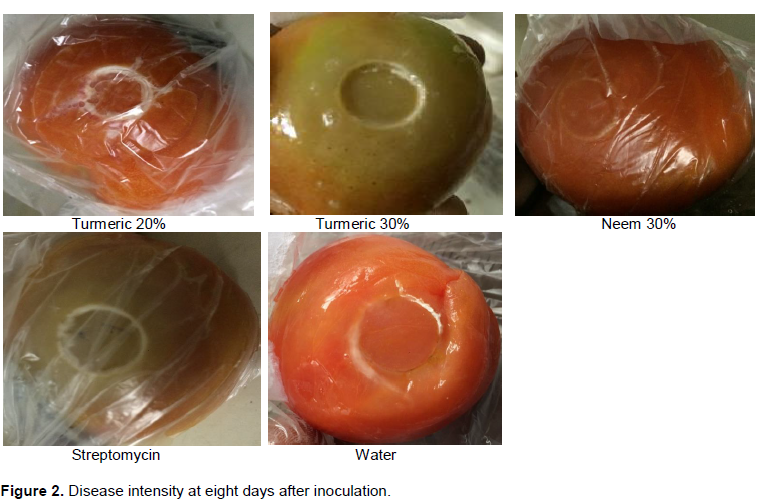
The highest intensity after four days of inoculation was recorded in coriander 30% which might have been due to effect of concentration which might possibly facilitated disease process, since concentrations were significant (Table 3 and Figure 2), while disease intensity was found to sharply increase at eight days after inoculation, with highest mean value recorded in ginger 30% (T2=46.2) and lowest value with streptomycin(T0b=27), followed by turmeric 20% (T3=27.6) then turmeric 30% (T4=27.8). Among the botanicals, the lowest disease intensity was with T3=27.6, T4=27.8, T1=32.0 and T6= 38.6 which did well when compared to both treated and untreated controls as they fall in between and more closer to the treated control (Table 3). This also agrees with the study of Akbar et al. (2014) findings.
Eight botanicals viz: Zingiber officinale, Curcuma longa, Azadirachta indica, Coriandrum sativum L., Allium sativum L., Citrus aurantifolia, Nigella sativa L. and Capsicum annuum each at two concentrations were screened in vitro along with treated (streptomycin sulphate) and untreated (sterile distilled water) controls, using disc inhibition zone. Out of these eight botanicals, four were selected based on their performance under the screening and used for evaluation of their efficacy on bacterial soft rot of storage tomato along with same controls. Significant results were obtained when eight botanicals were screened and the chosen four against the bacterial soft rot. In the present study, turmeric 30% (T4) proved to have highest potential to be used for the management of soft rot of tomato (Pectobacterium carotovora subsp. carotovora) disease compared to the rest botanicals.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed I (1976). Studies on the seed mycoflora and synchytrium disease of til (Sesamum indicum L.). Ph.D. Thesis, Agra University, Agra India.
|
|
|
|
Akbar A, Din Subhanud, Ahmad M, Khan G, Alam S (2014). Effect of Phytobiocides in Controlling Soft Rot of Tomato. J. Nat. Sci. Res. 4:11
|
|
|
|
|
Al-Samarrai G, Singh H, and Syarhabil M (2012). Evaluation of eco-friendly botanicals (natural plant products) as alternatives to synthetic fungicides. Ann. Agric. Environ. Med. 19(4):673-676.
|
|
|
|
|
Apisariyakul A, Vanittanakom N, Buddhasukh D (1995). Antifungal activity of turmeric oil extracted from Curcuma longa. J. EthnoPharmacol. 49:163-169.
Crossref
|
|
|
|
|
Bankole SA (1996). Effect of essential oils from two Nigerian medicinal plants (Azadirachta indica and Morinda lucida) on growth and aflatoxin B1 production in maize grain by a toxigenic Aspergillus flavus. Lett. Appl. Micro. 24(3):190-192.
Crossref
|
|
|
|
|
Bell K, Sebaihia S, and Pritchard M (2004). Genome sequence of the enterobacterialphytopahtogens, Pectobacterium carotovora subsp. atroseptica and characterization of virulence factors. Proc. Nat. Acad. Sci. U.S.A. 7:5-10.
|
|
|
|
|
Bhardwaj SK, Laura JS (2008). Antibacterial Activity of Some Plant Extracts against Pathogenic Bacteria Pectobacterium carotovora subsp. carotovora. Potato J. 35:1-2.
|
|
|
|
|
Charkowsky A.O (2006). The soft rot Erwinia. In: Gnanamanickam SS, ed. Plant-Associated Bacteria. Dordrecht, Netherlands: Springer. pp. 423–505.
Crossref
|
|
|
|
|
Czajkowski R, Perombelon MCM, Vanveen JA, Van der Wolf JM (2011). Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A Revew. Plant Pathol. 10:1365-3059.
Crossref
|
|
|
|
|
Govindachari TR, Sursh G, Gospalakrishnan G, Banumathy B, Masilamani S (1998). Identification of antifungal compounds from the seed oil of Azadirachta indica. Phytoparasitica 26(2):109-116.
Crossref
|
|
|
|
|
Kotoujansky A (1987). Molecular genetics of pathogenesis by soft rot Erwinia. Ann. Rev. Phytopathol. 25:405-430.
Crossref
|
|
|
|
|
Koul O, and Walia S (2009). Comparing impacts of plant extracts and pure allelochemicals and implications for pest control. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Res. 4(049):1-30.
|
|
|
|
|
Mahfuzul-Haque D, Bari ML, Inatsu Y, Juneja VK, Kawamot S (2007). Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodborne Pathogens Dis. 4(4):418-488.
|
|
|
|
|
Nauvov NA (1924). On the question of the possibility for determining the degree of plant infection by fungus parasites Trudy iv. Entomophytopath. Congr. Mascow 22:217-228.
|
|
|
|
|
Obongoya BO, Wagai SO, Odhiambo G (2010). Phytotoxic effect of selected crude plant extracts on soil-borne fungi of common bean. Afr. Crop Sci. J. 18(1):15-22.
Crossref
|
|
|
|
|
Opara E, Njoku T, Ogbonna U (2013). Control of postharvest bacterial diseases of tomato in Abia State, South Eastern Nigeria: J. Biol. Agric. Healthc. 3:19.
|
|
|
|
|
Pangtey YPS (1979). Investigation on seed born fungus of Dolichus biflorus L. and Glycine hispida maxinm. Two common legumes of Kumaun. Ph.D. Theses, Kumaun University, Nainital.
|
|
|
|
|
Paradza VM, Icishahayo D, Ngadze E (2012). Efficacy of botanical extracts from garlic and neem on control of potato soft rot pathogens. Uniswa J. Agric. 16(2):1-10.
|
|
|
|
|
Perombelon MCM, Kelman A (1980). Ecology of the soft rot Pectobacterium. Ann. Rev. Phytopahtol. 18: 316-387.
Crossref
|
|
|
|
|
Rachana S, Venugopalan P (2014). Antioxidant and bactericidal activity of wild turmeric extracts. J. Pharmacog. Phytochem. 2(6):89-94.
|
|
|
|
|
Rahman MM, Khan AA, Ali ME, Mian IH, Akanda AM, Abd Hamid SB (2012b). Botanicals to control soft rot bacteria of potato: Sci. World J. 2012:796472.
Crossref
|
|
|
|
|
Rashid MA, Singh DP (2000). A manual on vegetable seed production in Bangladesh. AVRDC-USDA, Bangladesh project. Available at: View
|
|
|
|
|
Rick CM (1969). Origin of cultivated tomato, current status of the problems, Abstract of Interna. Bot. Congr. P. 180.
|
|
|
|
|
Saleem MY, Akhtar KP, Asghar M, Iqbal Q, Rehman A (2011). Genetic control of late blight, yield and some yield related traits in tomato (Solanum lycopersicum L.). Pak. J. Bot. 43:2601-2605.
|
|
|
|
|
Silva F, Ferreira S, Qeiroz JA, Dominques FC (2011). Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 14:79-86.
Crossref
|
|
|
|
|
Slusarenko AJ, Patel A, Portz D (2008). Control of plant diseases by natural products; Allicin from garlic as a case study. Eur. J. Plant Pathol. 121(3):313-322.
Crossref
|
|
|
|
|
Subrahmanyam P, Mc Donold WF, Reddy LJ, Nigam SN, Gibbons RW, Rao RP, Singh AK, Pande S, Reddy PM, Rao PVS (1995). Screening methods and sources of resistance to rust and late leaf spot of groundnut. Patancheru 502 324, Andhra Pradesh, India. Info. Bull. P. 47.
|
|
|
|
|
Taylor IB (1986). Biosystematics of the tomato in the tomato. In: tomato crop. A scientific basis for improvement (eds. Artherton, J.G, and Rudich. J.). pp. 1-34.
|
|
|
|
|
Vavilov NI (1951). The origin, variation, immunity and breeding of cultivated plants. Chron. Bot. 13:1-366.
Crossref
|
|
|
|
|
Wood M (1998). Ubi 7-new tool for potato breeders. Agric. Res. Phytopathol. pp. 12-13.
|
|