ABSTRACT
A study was undertaken to determine the thermo-tolerance of 4 month old dwarf Cavendish banana plants of cultivar "Malindi" planted using suckers. The electrolyte leakage procedure was used to generate sigmoidal curves from which the thermo-tolerance was determined at 50% electrolyte leakage. A completely randomized design (CRD) with three replicates was used. Leaflets (1.2 cm width × 5 cm length) from third fully opened leaves of plants fertilized by composted dairy cow manure and mineral fertilizers were exposed to heat regimes ranging from 30 to 80°C (at 5°C increments) for 30 min and 23°C (room temperature) as control. The results revealed that there was a positive strong correlation between heat regimes and electrolyte leakage percentage (r2 = 0.73, p ≤ 0.01). Lethal temperature for Malindi banana plants was 58°C. The present investigation provided an accurate determination for heat tolerance of dwarf Cavendish banana cultivar "Malindi" and thus helps banana farmers to well manage their plants during such harsh weather conditions.
Key words: Dwarf Cavendish banana, electrolyte leakage, heat stress, thermo-tolerance.
It is well known that heat stress is a major factor limiting the productivity and adaptation of many crops, especially when temperature extremes coincide with critical stages of plant development. Due to the global climatic changes, high temperatures become one of the main constraints threatening and retarding the growth and production of crops and thus agricultural sustainability. Recently, banana cultivation in the Sultanate of Oman is suffering from such stresses. According to McWilliams (1980), Ingram and Buchanan (1984) and Lester (1985) that tolerant of plants to heat stresses depends on the varieties, period of the thermal exposure and stage of growth of the exposed tissue (McWilliams, 1980; Ingram and Buchanan, 1984; Lester, 1985). One of the most important components of plant heat stress tolerance is its ability to adapt to high temperature under field conditions (Chen et al., 1982). According to Levitt (1988) direct heat injury results from short exposure to extreme temperature and causes changes in the native structure of the plasma membrane components (lipids and proteins). As a result, membrane losses its semi permeability and becomes leaky and more electrolytes could easily leak out. One of the effective procedures to measure cell membrane thermo-stability as well as indicator to direct heat-injury is electrolyte leakage (EL) technique (Sullivan and Ross, 1972; Furmanski and Buescher, 1979; Martineau et al., 1979; Ahrens and Ingram 1988; Ingram and Buchanan, 1981; Sullivan and Ross, 1972; Heckman et al. 2002; Shi et al., 2006). The validity of this method has been approved by stress physiologists and is relevant to the tolerance level in the field or in-situ on different crops including banana (Marcum, 1998). In this regard, Ingram and Ramcharan (1988) studied the effect of heat stress (temperature regimes 30 to 60°C for 30-300 min) on roots of banana and rooted cuttings of Dracaena marginata plants. They found that the critical temperature for each plant decreased exponentially as electrolyte leakage increased. Predicted critical exposure times at 48 and 52°C were >300 and 221 ± 51 min, respectively, for Dracaena, and 225 ± 36 min and 105 ± 14 min for banana, respectively. Ingram et al. (1987) aimed at detecting the critical high root zone temperatures for Carrizo citrange (Citrus sinensis × Poncirus trifoliate) seedlings that were grown for 9 weeks at root-zone temperatures of 28, 34 or 40°C for 6 h daily. The shoot: root ratio was significantly increased at 40°C. Also, Ahrens et al. (1988) employed electrolyte leakage procedure to determine the differences in tolerance to direct heat injury to leaves of some citrus cultivars and discover that the lethal temperature for a 20 min exposure was 54.3 ± 0.5°C for ‘Glen’ citrange citrus variety and 56.1± 0.4°C for ‘Swingle’ citrumelo variety. However, Ingram et al. (1984) found that roots of “Swingle” citromelo died at 53°C. Xiao and Zhao (1990) conducted studies on physiological and biochemical indices of heat tolerance of citrus leaves by using electrolyte leakage procedure. Leaves of 5 citrus species were treated with warm water at temperatures of 36 and 66°C for 20 min exposure time. Their results indicated that sour orange followed by lemon had the highest degree of heat tolerance. Zhonghai et al. (1999) exposed tissues to different temperature regimes of 46, 48, 50, 52, 54, 56, 58 and 60°C and compared them with leaves treated at 35°C (control) for 20 min by using electrolyte leakage procedure. They found that the plasma membrane was damaged and the rate of leakage increased with temperature also Anderson et al. (1990) determined the capacity of pepper leaves to acclimatize to high temperature by using electrolytes leakage procedure. Their results showed an interaction between exposure to temperature and duration, as well as lethal temperature which decreased linearly from 53 to 46°C as exposure duration increased exponentially from 5 to 240 min. Plants grown at 22/20°C day/night cycles and held 24 h at 38/30°C had increased their heat tolerance by 35°C, and from 51 to 54°C. There is limited research on the application of electrolyte leakage (EL) procedure on banana plants. On the other hand, according to studies on thermo-tolerance of dwarf Cavendish banana group plants through leaflets have not been reported so far.
This laboratory experiment is aimed at closing this research gap. Additionally, providing such information will offer banana growers with accurate information about the favorable time of the year for transplanting banana plants and protecting them against heat in the field. The objective of this study was therefore, to determine the heat tolerance of Malindi banana cultivar under Omani weather conditions.
Third leave from the top of banana plant locally named Malindi cultivar was used. Middle part of the leaf blade was cut and rinsed in deionized water to remove the dusts and electrolytes adhering to the surfaces and lightly cleaned with tissue papers. Leaf segments of five centimeter length and 1.2 cm width were cut. One segment was placed in each test tube, and 1 ml of deionized water (< 0.2 ds m-1) was added to the test tube to prevent tissue desiccation and loosely covered with aluminum foil. All test tubes were placed in a water bath shaker equipped with thermostat and the tissues were exposed to a heat regime ranging from 30 to 80°C (5°C increments). The exposure time to each temperature was 30 min. Three tubes per treatment remained at 22°C as controls. At the end of each 30 min exposure, leaflet segments were removed from the water bath; cut into 1 mm strips to allow uniform diffusion of electrolytes, and returned to the tubes along with 40 ml of deionized water, and incubated in a refrigerator at 7°C overnight. This was followed by electrical conductivity determination of each solution. In the next day, leaf segments were taken out from the refrigerator, warmed up to room temperature (22 ± 2°C) and placed in a mechanical shaker (Gesellschaft Fur Labortechnik (GFL) mbh- model 3015-Germany) and shaken for one hour to diffuse the electrolytes. Thereafter, electrolytes leakage before final cells damage was measured with electrical conductivity meter (Orion-model 150-USA). Leaf segments were then fully damaged by autoclaving (Mediclave - Japan) (121°C) for 10 min and left on the shaker device for 1 h, then the total electrolyte leakage reading were taken by using the same conductivity meter. Percentage of electrolyte leakage before killing and after killing was calculated (Ahrens and Ingram, 1988) as follows:
EL% = [(electrolyte leakage before killing) / (electrolyte leakage before killing)] × 100
The three replicates of the control treatment leaflets went through the same procedure to find percentage of electrolyte leakage. The experiment consisted of twelve treatments, each treatment was replicated 3 times in a completely randomized design, and each leaflet represents one replication. Two-way analysis of variance was used to analyze the data of electrolyte leakage. Multiple comparisons among means were performed with Duncan's test (DMRT). Person's correlation analysis was used to examine relationships between heat regimes and electrolyte leakage percentage variables. All statistical analyses were carried out using GenStat Release 11.1. Sigmoid curves were illustrated by using Curve expert 1.3 programs.
Exposure of plasma membrane to direct heat stress resulted in changes the membrane permeability and loss its semi-permeability and thus electrolyte leakage from cells can occur. Determination of cell membrane thermostability in plant tissues by using cell electrolytes leakage technique have been widely used (Levitt, 1980; Ingram, 1986). Under heat stress, proteins of the plasma membrane become denatured or aggregated according to the severity of stress and/or membrane lipids become hyperfluid. These changes resulted into increased leakage of electrolytes from the membrane (Levitt, 1980).
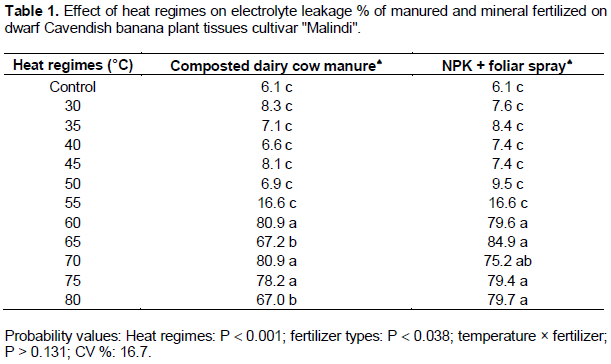
The results indicated that the injury occurred to plasma membrane of banana leaflet tissues as a result of direct exposure to heat regimes was gradually increased based on temperature degree. There were significant differences (p ≤ 0.05) between heat regimes on the percent of cell electrolytes leakage. As shown in (Figures 1 and 2), the shape of the sigmoidal curve was consistent. The lethal temperature was determined at 50% electrolyte leakage. The sigmoidal curve started with slow increase in electrolyte leakage at low temperatures and then increased rapidly at 55°C onwards. Beyond 50% electrolyte leakage, there was leveling off with very minor changes in this leakage with increase in the temperature. The sigmoidal pattern of electrolyte leakage obtained in this study, for heat stress (Figures 1 and 2) is in line with other previous studies (Ingram and Buchanan, 1988; Ahrens and Ingram, 1988; Nielsen and Orcutt, 1996). The figures clearly show inflection of the line. Thus, based on the inflection point at 50% electrolyte leakage, it was evident that the lethal temperature for 4 months old dwarfs Cavendish banana plants was 58°C. Our results are not in agreement with the report of Taylor and Sexton (1972) that thermal danger point for banana is 47°C as well as the report of Ingram and Ramcharan (1988) who found 48°C to be the lethal temperature for banana root. However, our results is in consonants with other researchers (Ingram and Buchanan, 1984; Lester, 1985) that genotype, period of exposure and growth stage of tissue play a vital role in determination of the level of plant tolerance to heat stress. Although there was a variation between low temperatures on the percent of electrolyte leakage, but statistically there was no significant difference among them (p ≥ 0.05). However, significant differences were detected between low and high temperature regimes (p ≤ 0.05). Also, it was found that leakage of electrolytes was similar in leaflets segments irrespective of fertilizer types used even at relatively low temperatures such as control, 30, 35, 40, 45, 50 and 55°C (Table 1). Similar results were obtained at high temperatures of 60, 65, 70, 75 and 80°C. Also, there was no interaction between temperature regimes and fertilizer on the percentage of electrolyte leakage. Regardless of the type of fertilizer used, the injury point to leaflet tissues of different fertilized plants started at the same temperature (55°C). Although leaflet tissues taken from plants that was fertilized with manure compost had significantly less electrolyte leakage when compared with those under mineral fertilizer (7% lower) (Table 1). This may be attributed to the ability of compost to supply nutrients for long time especially calcium and potassium which are very important in strengthening cell membranes.
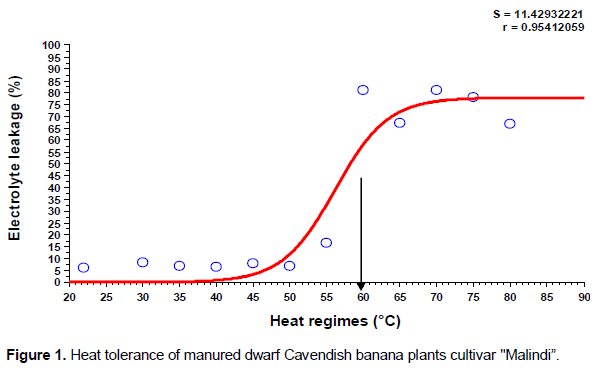
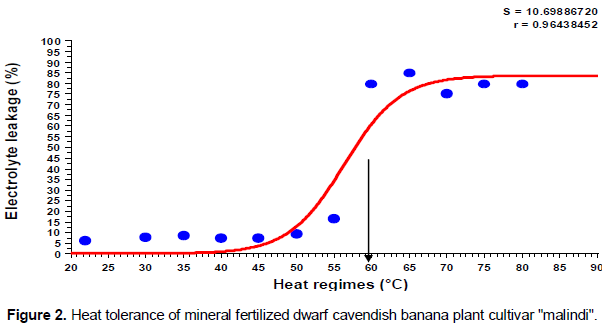
The sigmoidal pattern of electrolyte leakage obtained in this study, for heat stress is in the line with other previous studies (Ingram and Buchanan, 1988; Nielsen and Orcutt, 1996). The current study provided experimental evidence for heat tolerance of banana plants (Malindi cultivar). Perhaps this is the first study that provided a quantitative determination for heat tolerance of dwarf Cavendish banana plants by using leaflets segments through electrolyte leakage technique. Moreover, this information has very important implications since many banana plants could be affected or die if air temperature during the day reaches more than 40°C especially during summer months. Accordingly, in the present study, heat absorption factor has been considered because tissue temperature is usually higher than air temperature by at least 10 to 12°C (Levitt, 1988). These results would guide banana growers to the suitable time of the year before transplanting banana plantlets and protect their plants against extreme of temperatures.
Results presented in this investigation demonstrated that heat stress regime resulted consistently in generating a sigmoidal curve of electrolyte leakage. It provides evidence of the validity of electrolyte leakage technique in determining heat tolerance of banana plants (Malindi cultivar) throughout leaf tissues. This study showed perhaps for the first time a quantitative determination of the thermo-tolerance of Dwarf Cavendish banana plants (Malindi cultivar). Although average percent of electrolyte from plant tissues that received compost dairy manure was less than that fertilized by mineral fertilizers, but heat tolerance of leaflets tissues obtained from either plants receiving organic composted dairy manures or mineral fertilizers was quantitatively the same. Lethal heat tolerance for dwarf Cavendish banana plants was found to be 58°C. The duration factor in stress is very important and it should be taken in to consideration in future studies.
The authors have not declared any conflict of interest.
The author thanks the International AL-Batinah Farm for supplying banana suckers and Oman Agri-fertilizer CO.LL.C for providing composted cow manures. Also, special thanks to Eng. Zahir Al-Faree for his assistance in conducting this experiment in the laboratory.
REFERENCES
|
Ahrens MJ, Ingram DL (1988). Heat tolerance of citrus leaves. Hortic. Sci. 23:747-748. |
|
|
|
Anderson AJ (1990). Production of methanol from heat-stressed pepper and corn leaf disks. J. Am. Soc.119:468-472. |
|
|
Chen HH, Shen ZY, Li PH (1982). Adaptability of crop plants to high temperature stress. Crop Sci. 22(4):719-725.
Crossref |
|
|
|
Furmanski RJ, Buescher RW (1979). Influence of chilling on electrolyte leakage and internal conductivity of peach fruits. Hortic. Sci. 14:167-168. |
|
|
Heckman NL, Horst GL, Gaussoin RE, Tavener BT (2002). Trinexapac-ethyl influence on cell membrane thermostability of Kentucky bluegrass leaf tissue. Hortic. Sci. 92:183–186.
Crossref |
|
|
|
Ingram DL, Buchanan D (1981). Measurement of direct heat injury of roots of three woody plants. Hortic. Sci.16:769-771. |
|
|
|
Ingram DL, Buchanan D (1984). Lethal high temperatures for roots of three citrus rootstocks. J. Am. Soc. 109:189-193. |
|
|
|
Ingram DL (1986). Root cell membrane heat tolerance of two dwarf hollies. J. Am. Soc. Hortic. Sci. 111:270-272. |
|
|
|
Ingram DL, Ramcharan C (1988). Grande Naine' banana and Dracaena marginata 'Tricolor' root cell membrane heat tolerance fruits. Crop Sci. 43:29-33. |
|
|
|
Ingram DL, Ramcharan C, Buchanan DW (1987). Critical high root-zone temperatures for container-grown. Citrus Proc. Florida State 99:214-217. |
|
|
|
Lester GE (1985). Physiology of melon leaf membrane thermostability during heat conditioning. J. Am. Soc. 111:561-564. |
|
|
|
Levitt J (1980). Response of plants to environmental stresses. volume 1. Chilling Freezing and high temperature stresses. New York: Academic Press. |
|
|
Marcum KB (1998). Cell membrane thermostability and whole plant heat tolerance of Kentucky blue grass. Crop Sci. 38:1214-1218.
Crossref |
|
|
|
Martineau JR, Specht JE, Williams JH, Sullivan CY (1979). Temperature tolerance in soyabeans.1. Evaluation of technique for assenting cellular membranes thermostability. Crop Sci. 19:75-78. |
|
|
|
McWilliams JR (1980). Adaptation of plants to water and high temperature stress: summery and synthesis- adaptation to high temperature stress. pp. 444-447. |
|
|
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006). Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 48:127–135.
Crossref |
|
|
|
Sullivan CY, Ross WM (1972). Selecting for drought and heat resistance in grain sorghum. In Mussell, H. and Staples, R. Stress Physiology in Crop Plants. pp. 263-281. |
|
|
|
Xiao SY, Zhao DZ (1990). Preliminary studied on physiological and biochemical indices of heat tolerance of citrus leaves. J. Fruit Sci. 7:217-220. |
|
|
|
Zhonghai S, Ma-xiang T, Sun ZH (1999). Study on the thermostability of plasma membrane in citrus leaves. J. Huazong Agric. Univ. 18:375- 377. |