ABSTRACT
Gray mold and soft rot are the most important postharvest diseases of tomato worldwide. A survey of fresh-market tomato fruit was conducted in Oahu to determine which fungal and bacterial pathogens were most commonly associated with postharvest disease. Alternaria, Botrytis, Colletotrichum, Fusarium, Geotrichum, Mucor, Stemphyllium, Rhizopus and Penicillium were the most frequently isolated fungi and Acetobacter, Gluconobacter, Klebsiella, Leuconostoc and Pectobacterium were the prevalent bacteria. Fifty-one percent of the diseased tomatoes had been imported from California and Mexico and 49% had been grown locally at three sites in Oahu. Pathogenicity tests revealed that 33 of 99 fungal isolates and 10 of 17 bacterial isolates were pathogenic on tomato types known as common market, cherry and grape tomato. Based on fruit assays, Botrytis cinerea (B03) and Pectobacterium carotovorum (BA17) were the most virulent isolates. Tested leaf extracts of Capsicum annuum cv. Stocky Red, C. annuum cv. Criolla de cocina, Capsicum chinense cv. NuMexsuave, Tagetes tenuifolia, Aloe vera, Origanum vulgare and Azadirachta indica were ineffective as biopesticides and did not reduce spore germination or mycelial growth of B. cinerea (B03) nor P. carotovorum (BA17). In contrast, a proprietary product (PF) reduced mycelial growth of B. cinerea (B03) and was further evaluated at doubling concentrations ranging from 0.0625 to 1 ml/L. Mycelial growth of B. cinerea and other fungi was completely inhibited by exposure to PF at 1 ml/L. On the other hand, PF was not an effective biopesticides against P. carotovorum. PF shows promise for reducing gray mold and will be evaluated as a preharvest spray on tomato plants in the greenhouse.
Key words: Survey, postharvest diseases, tomato, natural product, Botrytis cinerea, Pectobacterium carotovorum.
Public concern about fungicide residues on raw fruits and vegetables has stimulated research efforts using natural products to reduce incidence of postharvest diseases. Approximately, 25 and 38% of harvested fruits and vegetables, respectively, are lost to postharvest spoilage in the U.S. and global markets (Kantor et al., 1997). Fresh fruit and vegetables can be infected by pathogenic fungi and bacteria during crop growth in the field,
harvesting, postharvest, storage and consumption (Barth et al., 2009). Postharvest diseases cause economic losses in field because of added costs of harvesting, transportation and storage (Adikaram, 1986). The current study focuses on tomato (Solanum lycopersicum Mill), which is one of the most important vegetables produced globally, comprising approximately 14% of world vegetable production (Kader, 2004; FAO, 2003). The production value of tomato, estimated at more than $50 billion, makes it the fourth most important commercial crop in the world (Vincent et al., 2013). Tomato is also one of the leading vegetable crops in Oahu, Hawaii. The first objective of this work was to determine the most serious postharvest diseases of tomato in Oahu and to identify the most virulent pathogens associated with each disease. The second objective was to evaluate natural products for biopesticidal activity against pathogenic fungi and bacteria associated with tomato postharvest diseases.
Survey of postharvest diseases in tomato fruit
A survey was conducted in Oahu, Hawaii extending from November 2014 to April 2015. Samples of infected tomato were randomly collected from 17 locations and 37 markets in Oahu. Two samples (each sample consisting of 10 fruits) were selected from each market. Tissues showing symptoms of postharvest disease were cultured to identify associated pathogens. The percentage of infected tomato based on origin (local or imported) was reported.
Isolation and identification of pathogens
Small (1 cm) sections of infected fruit were cut and surface-sterilized individually in 2% sodium hypochlorite for 1 min and rinsed twice in sterile distilled water. The pieces were dried between sterile Whatman No.1 filter paper and cultured on water agar plates and incubated at 28±2°C for 24 h. Single hyphal tips were transferred to Petri dishes containing V8 medium and incubated at 28±2°C for 5 days under a 12 h photoperiod (Carisse and Van Der Heyden, 2015). Purified cultures were visually identified utilizing laboratory manuals (Dugan, 2006). For bacterial isolates, small sections of rotted tissues were suspended in distilled water for two minutes and the suspension was streaked onto the surface of nutrient agar (NA) plate and plates were incubated at 30°C for 24 h. Basic bacteriological tests including KOH sensitivity, oxidation/fermentation (OF), production of catalase, degradation of sodium polypectate, and hydrolysis of esculin and starch were conducted on each isolated bacteria. All bacterial strains were maintained in freezers (-80°C) until used. Presumptive identifications were confirmed with 16S rDNA sequence analysis (Weisburg et al., 1991).
Pathogenicity tests
Pathogenicity tests were performed on all fungal and bacterial isolates on three types of tomato fruits as previously described by Ahmed et al. (2016). Fruits were selected to be uniform in size and color, free from wounds and showing no symptoms of disease. Fruit were washed with tap water, surface sterilized by dipping in 1% sodium hypochlorite solution for 10 min, rinsed by dipping twice in sterile distilled water for at least 10 min, and dried in ambient air. A wound (1 mm diameter in 4 mm deep) was made on each fruit using a pipette tip. Mycelial plugs from 10-day-old-cultures of the fungal isolates were inserted into wounds using 0.2 to 10 µl pipette tips. For bacterial stains, fruit were inoculated with 20 µl of a bacterial suspension (1x108/CFU). Inoculated fruit were placed in plastic box containing sterile paper towels moistened with sterile water and incubated for 72 h at 23°C. An organism was recorded as pathogenic if symptoms of rot appeared on the tested fruit. The experiments were set up separately for fungal and bacterial isolates with four replications and each experiment was repeated twice.
Virulence tests
Tests were conducted to determine the most virulent isolates on each of the three types of tomato fruit (common market, cherry and grape). Fruits selected were uniform in size and color, free from wounds and showing no symptoms of disease. Virulence of each isolate was determined by measuring the lesion diameter of inoculated fruit after incubation at 23°C for 72 h. The experiments were set up as complete randomized design (CRD) with four replicates. Data were analyzed using SAS 9.2 V.USA and means were compared by Duncan’s multiple range tests. Differences at p <0.05 were considered significant. The tests were repeated twice.
Molecular identification
Fungal DNA was extracted from freshly collected mycelium of 10-day-old cultures using the Microbial DNA Isolation Kit (MO BIO, Laboratories, Inc.). The ITS region of the fungal isolates was amplified with the primer pair ITS3 (5-GCA TCG ATG AAG AAC GCA GC-3) and ITS4 (5- TCC TCC GCT TAT TGA TAT GC-3) (Nikolcheva et al., 2003).
Bacterial DNA was extracted from overnight cultures using the Microbial DNA Isolation Kit (MO BIO, Laboratories, Inc.) according to manufacturer's instructions. The 16S rRNA was amplified by PCR for all the isolates using the primers: 16S forward primer (5'-AGAGTTTGATCCTGGCTCAG-3) and 16S reverse primer (5′ACGGCTACCTTGTTACGACTT-3′). PCR was performed as previously described by Srinivasa et al. (2012) and Weisburg et al. (1991). Each PCR reaction was run with a negative control (no DNA). The PCR products were electrophoresed on 1.5% agarose gels, stained with 0.4 µg/ml ethidium bromide, and bands visualized with a UV illuminator.
Sequence analysis
Sequence analysis was conducted as described in previous work (Ahmed et al., 2016). PCR product was cleaned utilizing ExoSAP-1T (Affymetrix, Inc., USA). The 5 µl of post-PCR reaction and 2 µl ExoSAP-IT reagents were mixed. The mix was incubated at 37°C for 15 min followed by incubation at 80°C for 15 min. Each purified template was sequenced on both strands using two flanking primers (ITS3- ITS4) for fungal isolates and 16s primers for bacteria. The sequences of ITS 3 and 4 regions, 16s of the tested isolates were edited in order to generate a consensus sequence from forward and reverse sequence in the amplicon using sequence assembly software (DNA BASER). A consensus sequence was analyzed by NCBI BLAST database for fungal and bacterial identities.
Natural controls
Leaf extracts made from Capsicum annuum cv. Stocky Red, Capsicum annuum cv. Criolla de cocina, Capsicum chinense cv. NuMexsuave, Tagetes tenuifolia, Aloe vera (leaves and gel), Origanum vulgare and Azadirachta indica (Neem oil) and a proprietary formulation (PF) (Agrichem, Inc., Australia) were tested. Leaves were extracted by following method described by Wilson et al. (1997)with some modification. Raw leaves were collected in plastic bags and freeze for a minimum of 12 h at 20°C. Plastic bag was removed and leaves fluid were sterilized by using 0.22 µm Millipore filter and stored in 4°C until used.
Assays of antifungal activity
The antifungal activity of nine plant extracts was evaluated against Alternaria and Botrytis isolates by using an inhibition assay described as the ‘poisoned food method’ (McCutcheon et al., 1994)with slight modification. A 5 ml sterilized crude extract was mixed with 15 ml of 45°C cooled molten V8 medium and allowed to solidify at room temperature for 30 min. A mycelial disc 6 mm diameter of 7 to 10-day-old cultures was transferred to a Petri plate containing V8 and crude extract. The V8 plate without plant extract served as a control. The antifungal activity of PF was evaluated against B. cinerea (B03) at five concentrations 1, 0.5, 0.25, 0.125, 0.0652 ml/L. The most effective PF concentration was reevaluated for the remaining 33 pathogenic fungal isolates. Since different genera have different growth rates on V8 medium, a separate control was established for each fungus by recording the time needed for mycelium to reach the edge of the Petri dish. At that point, the corresponding plate containing PF was measured for inhibition. The experiments were conducted as complete randomized design (CRD) with four replications. Data were analyzed using SAS 9.2 V.USA. Means were compared by Duncan’s multiple range tests. Differences at p <0.05 were considered significant. Each experiment was repeated three times.
Survey
Ninety nine fungal and seventeen bacterial isolates were recovered from tomato from the 37 markets in Oahu (Figure 1). Fungal genera were Alternaria, Botrytis, Colletotrichum, Fusarium, Geotrichum, Mucor, Stemphylium, Rhizopus and Penicillium. Bacterial genera were Acetobacter, Gluconobacter, Klebsiella, Leuconostoc and Pectobacterium (Figure 2 and Table 1). Examination of diseased tomatoes based on origin showed 51% were imported from California and Mexico and 49% were grown locally at three sites in Oahu. Some of these pathogens are known to survive on fruit and be spread during transportation, handling and storage (Barkai-Golan, 2001).
Pathogenicity and virulence
The pathogenicity tests showed that 33 of 99 fungal isolates and 10 of 17 bacterial isolates were pathogenic on all three types of tomato fruit (Table 2). Other reports indicate that fungi and bacteria survive and grow saprophytically on tomato (Smilanick, 2004; Agrios, 2005). In this study, the fungal isolates varied in virulence. The pathogenic isolates of B. cinerea were highest in lesion diameter range with 16-70, 10-32 and 10-25 mm on three types of tomato fruit common market, cherry and grape, respectively (Table 3). In addition, B. cinerea isolates were varied in their standard deviations, indicating that Botrytis isolates are different in virulence level. On the other hand, B. cinerea (B03) and P. carotovorum (BA17) were significantly more virulent than other isolates when tested on common market, cherry and grape tomato (Tables 4 and 5). Differences in virulence among pathogens are frequently a result of the differences in production of cell wall degrading enzymes (CWDEs), oxalic acid and/or secretion of pathogenicity factors (Bellincampi et al., 2014; Kubicek et al., 2014). All Botrytis and Pectobacterium isolates were pathogenic to the original host from which they were isolated (Figure 2). B. cinerea (B03) and P. carotovorum (BA17) produced significantly larger lesion diameters (Tables 4 and 5). In previous studies, Botrytis and Pectobacterium were some of the most important pathogens causing spoilage decay on tomato (Ahmed et al., 2016; Akbar et al., 2013; Etebu et al., 2013; Fillinger and Elad, 2015).
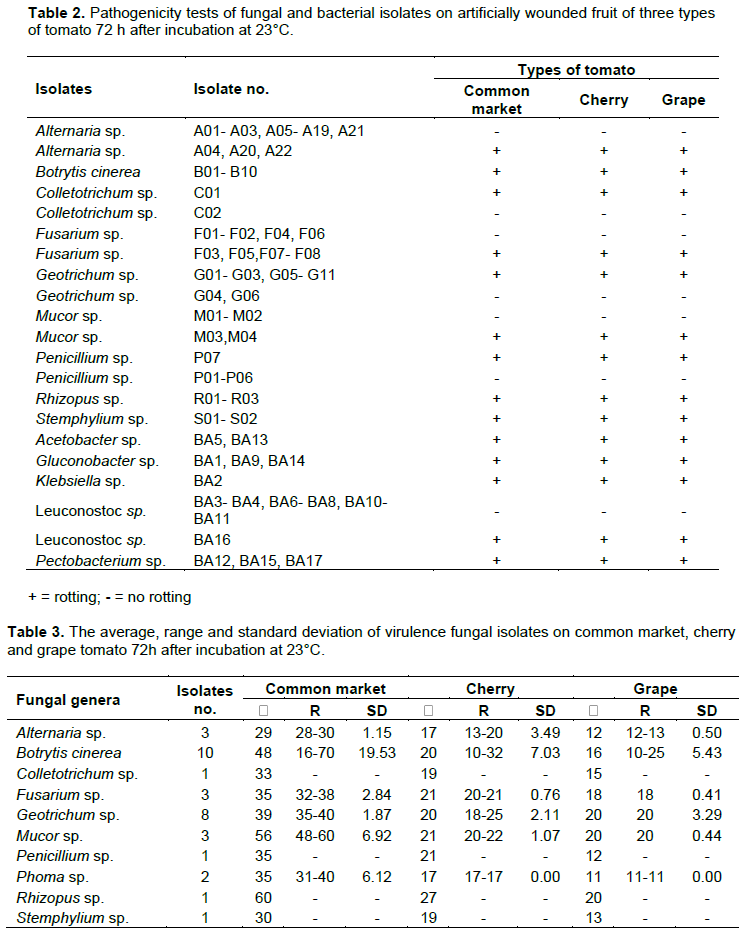
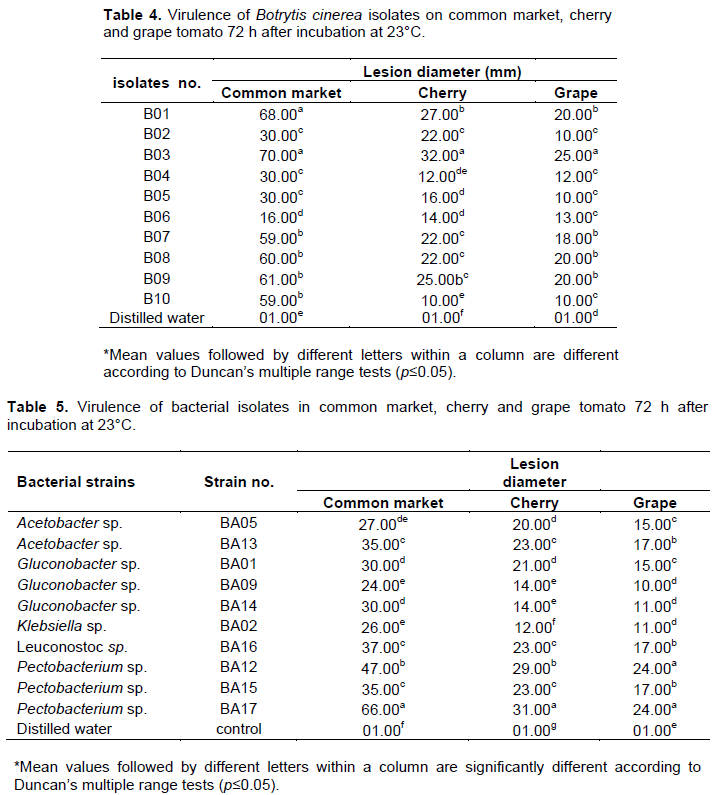
Molecular identification
APCR product of 370 bp was amplified efficiently for all fungal isolates. The ITS3-ITS4 region of identified fungi at > 98% similarity was compared with NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). B. cinerea was counterpart at 100% similarity. A PCR product with expected size 1400 bp was amplified for all bacterial isolates and NCBI BLAST of the 16S region identified bacteria at 99 to 100%. Pectobacterium isolates matched 100% with P. carotovorum.
Inhibitory effect of plant extracts on fungal colony
The crude leaf extracts of C. annuum cv. ‘StockyRed’, C. annuum cv. ‘Criolla de cocina’, C. chinense cv. ‘NuMexsuave’, T. tenuifolia, A. vera, O. vulgare and A. indica Neem oil showed no measurable inhibition of mycelial growth for Alternaria sp. or Botrytis sp. In contrast, PF completely inhibited mycelial growth of both fungi (Figure 3). The most effective PF concentration was 1 mL/L that completely inhibited growth of all 33 of the other tested pathogenic fungi (Table 6).
B. cinerea and P. carotovorum were the most virulent postharvest pathogens of tomato in Oahu. Thirty percent of the fungal and 58% of the bacterial isolates were pathogenic. A natural proprietary product (PF) had sufficient antifungal activity to completely inhibit mycelial growth of all isolated fungi but had no effect on the bacteria. This natural product is a potential alternative to synthetic fungicides in reducing postharvest gray mold disease.
The authors have not declared any conflict of interests.
REFERENCES
|
Adikaram N (1986). A survey of post-harvest losses in some fruits and vegetables and the fungi associated with them. Ceylon J. Sci. Biol. Sci. 20:1-10.
|
|
|
|
Agrios GN (2005). Plant pathology (Vol. 5): Elsevier Academic Press, Massachusetts 922 p.
|
|
|
|
|
Ahmed FA, Sipes BS, Alvarez AM (2016). Natural products to control postharvest gray mold of tomato fruit-possible mechanisms. J. Plant Pathol. Microbiol. 7:1-7.
Crossref
|
|
|
|
|
Akbar A, Din S, Ahmad M, Khan G, Alam S (2014). Effect of Phytobiocides in controlling soft rot of tomato. J. Nat. Sci. Res. 4:2225-09921.
|
|
|
|
|
Barkai-Golan R (2001). Postharvest diseases of fruits and vegetables: development and control: Elsevier 422 p.
|
|
|
|
|
Barth M, Hankinson TR, Zhuang H, Breidt F (2009). Microbiological spoilage of fruits and vegetables Compendium of the microbiological spoilage of foods and beverages. Springer pp. 135-183.
Crossref
|
|
|
|
|
Bellincampi D, Cervone F, Lionetti V (2014). Plant cell wall dynamics andwall-related susceptibility in plant–pathogen interactions. Front. Plant Sci. 228:1-8.
|
|
|
|
|
Carisse O,Van Der Heyden H (2015). Relationship of airborne Botrytis cinerea conidium concentration to tomato flower and stem infections: A threshold for de-leafing operations. Plant Dis. 99:137-142.
Crossref
|
|
|
|
|
Dugan FM (2006). The Identification of Fungi: An Illustrated Introduction with Keys, Glossary, and Guide to Literature: American Phytopathological Society 184 p.
|
|
|
|
|
Etebu E, Nwauzoma A, Bawo D (2013). Postharvest spoilage of tomato (Lycopersicon esculentum Mill.) and control strategies in Nigeria. J. Biol. Agric. Healthcare 3:51-61.
|
|
|
|
|
Food and Agriculture Organization of the United Nations (2003). Summary of food and agricultural statistics. Rome, Italy.
|
|
|
|
|
Fillinger S, Elad Y (2015). Botrytis-the Fungus, the Pathogen and Its Management in Agricultural Systems: Springer 486 p.
|
|
|
|
|
Kader AA (2004). Increasing food availability by reducing postharvest losses of fresh produce. Paper presented at the V International Postharvest Symposium. 682 2004 Jun 6, pp. 2169-2176.
|
|
|
|
|
Kantor LS, Lipton K, Manchester A, Oliveira V (1997). Estimating and addressing America's food losses. Food Rev. 20:2-12.
|
|
|
|
|
Kubicek CP, Starr TL, Glass NL (2014). Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Ann. Rev. Phytopathol. 52:427-451.
Crossref
|
|
|
|
|
McCutcheon A, Ellis S, Hancock R, Towers G (1994). Antifungal screening of medicinal plants of British Columbian native peoples. J. Ethnopharmacol. 44:157-169.
Crossref
|
|
|
|
|
Nikolcheva LG, Cockshutt AM, Bärlocher F (2003). Determining diversity of freshwater fungi on decaying leaves: comparison of traditional and molecular approaches. Appl. Environ. microbiol. 69(5):2548-2554.
Crossref
|
|
|
|
|
Smilanick JL (2004). Postharvest Diseases of Fruits and Vegetables: Development and Control: R. Barkai-Golan, Elsevier, Amsterdam 418 p.
Crossref
|
|
|
|
|
Srinivasa C, Sharanaiah U, Shivamallu C (2012). Molecular detection of plant pathogenic bacteria using polymerase chain reaction single-strand conformation polymorphism. Acta. Biochim. Biophys. Sin. 44:217-223.
Crossref
|
|
|
|
|
Vincent H, Wiersema J, Kell S, Fielder H, Dobbie S, Casta-eda-Álvarez NP, Maxted N (2013). A prioritized crop wild relative inventory to help underpin global food security. Biol. Conserv. 167:265-275.
Crossref
|
|
|
|
|
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991). 16S ribosomal DNA amplification for phylogenetic study. J. Bacterial. 173:697-703.
Crossref
|
|
|
|
|
Wilson CL, Solar JM, Ghaouth AEE, Wisniewski (1997). Rapid evalution of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 81:204-210.
Crossref
|
|