ABSTRACT
Infection of Citrus tristeza virus (CTV) in Assam was detected by double antibody sandwich-enzyme-linked immuno-sorbent assay (DAS-ELISA). Field surveys were carried out in 8 citrus growing districts of Assam (Tinsukia, Lakhimpur, Jorhat, Kokrajhar, North Cachar Hills, Karbi Anglong, Golaghat and Kamrup). Altogether, 411 samples were collected from three different citrus species, viz., Assam lemon (Citrus limon), Gul nemu (Citrus jambhiri) and Khasi mandarin (Citrus reticulata) to test against CTV. Results of biological indexing showed Kagzi lime (Citrus aurantifolia) to be the better indicator plant (symptom expression 60%) than Assam lemon (symptom expression 30%). The symptom expression on the indicator host was observed from 5th to 9th week after inoculation. The inoculated plants again tested after 9th week using DAS-ELISA assay. The results revealed that percentage of positive plants were 90% in Kagzi lime followed by Assam lemon (80%). This indicated that even the symptomless plants after inoculation give the positive reactions in DAS- ELISA assay. 261 samples were found to be infected using ELISA with polyclonal antisera to CTV. The species Assam lemon (C. limon) were found to be susceptible to CTV with estimated disease incidence up to 76.47% followed by Khasi mandarin (C. reticulata; 61.18%) and Gul nemu (C. jambhiri; 52.03%). Different age groups (<10, 10 to 15 and >15 years) of the citrus trees indicated that prevalence of CTV was more in older plants.
Key words: ELISA, Citrus tristeza virus (CTV), Assam lemon, Khasi mandarin, Gul nemu.
Citrus is considered to be one of the most remunerative fruit crops of India, having a lasting niche in the international trade and world finance. Most of the Citrus species are believed to be native to tropical and subtropical regions of Southeast Asia, particularly Northeastern India and the region between China and India (Ghosh, 2007). The Northeastern region of India is considered as the home of mandarin orange and many citrus fruits (Ghosh, 2007). Sub mountain and hilly tracts of states of Northeast, Assam, Meghalaya, Manipur, Arunachal Pradesh, Mizoram, Nagaland, Tripura, Sikkim and the Darjeeling hills of West Bengal grows excellent quality citrus fruits like, mandarin (Darjeeling, Khasi and Sikkim mandarin), sweet orange, lemons and limes. Khasi mandarin is the most important commercial cultivar of Northeast India, followed by Assam lemon, which is very popular in homestead gardens. Apart from these two cultivars, rough lemon (Citrus jambhiri Lush.), citron (Citrus medica L.), and sweet orange (Citrus sinensis Osbeck) are also grown in this region (Verma and Ghosh, 1979).
The global problem of citrus decline warranted special attention of agricultural scientists during the past few decades. The tristeza virus and viroid exocortis greening is caused by a fastidious bacterium in association with some fungi, nematodes and bacteria have been found to be involved in the citrus die back complex in India. Among the seven aphid species reported as vectors of Citrus tristeza virus (CTV), Toxoptera citricida has been found to be most efficient vector (Capoor and Rao, 1967). CTV is a phloem limited 2000 nm long filamentous virus having single stranded RNA genome (Karasev et al., 1995). The virus is genetically and biologically diverse and can cause field symptoms ranging from vein clearing, stem pitting, yellowing, slow decline and quick decline, or no symptoms depending on virus isolate, time of infection, root stocks, citrus cultivars and environmental conditions (Bar-Joseph et al., 1989).
Extremely limited information and experimental data are available on the occurrence and spread of CTV in the Northeastern region of India. However, a survey and indexing results recorded presence of tristeza in Khasi orange, Kagzi lime and Assam lemon in the Northeastern region in India (Bhagabati et al., 1989). The biological-indexing is relatively less reliable and time consuming, but double antibody sandwich-enzyme-linked immuno-sorbent assay (DAS-ELISA) has revolutionized the detection test, making it feasible to test large number of samples (Bar-Joseph et al., 1979; Cambra et al., 1979; Chakraborty and Ahlawat, 2001). In recent years, PCR based diagnosis has been used for the rapid diagnosis of citrus virus (Biswas, 2010; Edson et al., 2008). ELISA test can provide rapid, sensitive and economical detection of CTV in crude extracts from citrus trees.
The viral infection in plants alters the metabolism and physiology of the plant; hence, it is necessary to conduct an indexing of the viral disease to analyze the percentage of infection to control the disease in future. Diagnosis based on visible symptoms is generally unreliable and thus advanced diagnostic tools like ELISA and RT-PCR that has been used earlier in India (Chakraborty et al., 1992; Biswas, 2008), are very much essential for detection of CTV accurately. In the present study, advanced diagnostic tool that is, ELISA have been employed for detection of CTV. A comparative study on traditional method of diagnosis (that is, biological indexing) and antibody based detection technique (that is, DAS-ELISA) have been conducted and evaluated their efficiency.
Survey of Citrus tristeza virus (CTV) infection and aphid population
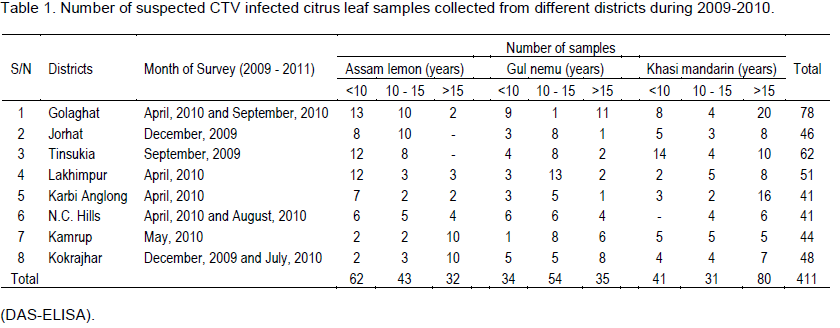
Survey and investigation was conducted in four agroclimatic zones viz., Upper Brahmaputra Valley zone, Lower Brahmaputra Valley zone, North Bank Plain zone and Hills zone of Assam covering eight districts (Jorhat, Tinsukia, Golaghat, Lakhimpur, Karbi Anglong, North Cachar Hills, Kamrup and Kokrajhar) during 2009-2010. Three to five locations from each district were surveyed for CTV infected plants. Suspected CTV infected samples from Khasi mandarin (Citrus reticulata), Gul nemu (C. jambhiri) and Assam lemon (Citrus limon) were collected from both commercial and home stead gardens of citrus trees from the age group of <10 years, 10 to 15 years and above 15 years trees. Altogether, 411 leaf tissue samples were collected from three different citrus species (Table 1).
Although symptoms of greening were also observed in all the surveyed locations on the investigation primarily concentrated on the CTV infection. There may be a mixed infection of greening and tristeza because symptoms of both the diseases were prominent in almost all the locations. But for further confirmation of greening, disease molecular assay has to be performed (Figures 1 and 2).
Biological indexing
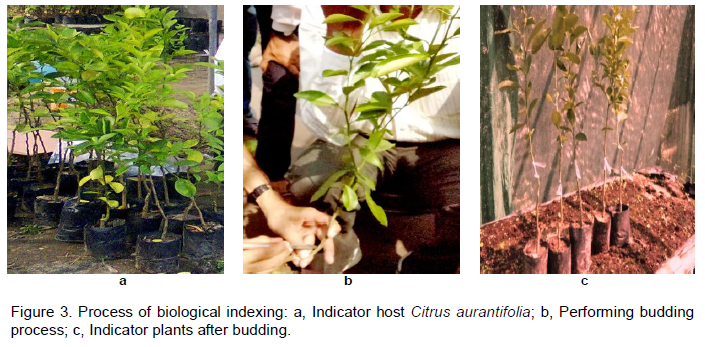
Bud wood from 30 trees, chosen at random and not necessarily showing symptoms, was used for indexing by the method of Roistacher (1991). Two indicator seedlings (Citrus aurantifolia that is, Maxican lime or Kagzi lime and C. limon to test as indicator hosts against CTV) were inoculated with buds obtained from CTV infected plants which were already tested as CTV positive using ELISA following the technique used by Wisler et al. (1996). Plants were grown in a greenhouse at about 27°C and inspected at least once each week for symptoms, beginning 15 days after inoculation. One bud was grafted onto each of the indicator plants. The plants were monitored for the symptom expression such as leaf yellowing and vein clearing. Fifteen (15) numbers of 1 year old seedlings of Assam lemon (C. limon) and 15 numbers of Kagzi lime or Maxican lime (C. aurantifolia) seedlings were grown inside the green house. Seedlings were tested by DAS-ELISA to test CTV negativity and were maintained in the greenhouse. The bud wood of 3 to 4 mm in diameter was budded at 20 cm height to each of the indicator seedlings. CTV infected scion of pencil thickness were collected from different fields nearby Jorhat, Assam. Trees from which bud sticks were taken were pre-tagged with CTV positive through DAS-ELISA. The samples were collected in the polypropylene bags. The scions were selected in such a way that every scion consisted of at least 2 to 3 swollen buds. Before putting the scion samples in polypropylene bags, these were wrapped in water soaked cotton so that they remain fresh during transportation. Then they were kept in refrigerator at 4°C until budding was performed and the process was completed within 48 h of their collection (Figure 3).
Double antibody sandwich-enzyme-linked immuno-sorbent assay (DAS-ELISA)
The leaf midrib tissue samples from different citrus growing areas were tested using DAS-ELISA as described by Clark and Adams (1977). The antibodies were obtained from Bioreba AG, CH4153, Reinach BL1, Switzerland. CTV IgG (20 µl) were diluted in 20 ml of coating buffer to coat each 96 well ELISA plate at the rate of 200 µl per well. The plate was incubated for 4 h at 30°C followed by three times washing with phosphate buffered saline Tween 20 (PBS-T). One gram (1 g) of leaf vein tissue from each sample was ground using mortar and pestle in 1 ml PBS-T. A 200 µl of the sample were added to each well. The plates were incubated over night at 4°C. ELISA plate was then washed three times with PBS-T. Following this, the enzyme conjugate was added to each well and the plate was then incubated for 4 h at 30°C. The plate was then washed three times with PBS-T. After washing, 5 mg p-nitrophenol phosphate substrate tablets were dissolved 20 ml of diethanolamine buffer and 200 µl of substrate was added in each well and observed for change of colour in the wells. CTV IgG, enzyme conjugate and substrates were purchased from Bioreba (USA). ELISA plate was then read by the ELISA plate reader (Bio Rad) using 405 nm wavelength after 1 h of addition of substrate. Plants were considered infected with CTV if the ELISA reading was four times higher the average reading of the healthy samples (usually ≥0.1) (Azzam et al., 2001). Data were analyzed and disease incidence was recorded.
Biological indexing
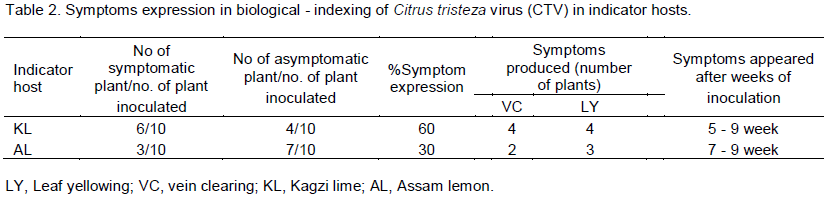
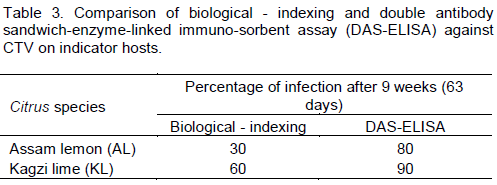
The results of biological indexing assay on two indicator hosts viz., Kagzi lime or Maxican lime (C. aurantifolia) and Assam lemon (C. limon) are shown on Tables 2 and 3. Both leaf yellowing and vein clearing symptoms were observed on both the hosts from 5th to 9th weeks after inoculation (Table 2). The results revealed that among the two species of citrus used as indicator hosts, Kagzi lime (C. aurantifolia) showed better expression of symptoms (60%) compared to Assam lemon (30%). The inoculated plants after 9th week (63 days) again tested using DAS-ELISA assay. The results revealed that percentages of positive plants were 90% in Kagzi lime followed by Assam lemon (80%). The comparison analysis between biological indexing and immunological indexing further indicated that although biological indexing was accepted as one of the reliable method of virus identification, the DAS-ELISA assay had been found more accurate and quick in confirming virus incidence (Table 3).
Identification of plant tissues as source of virus
DAS-ELISA of leaf samples from eight districts of Assam on three different Citrus species was performed successfully and ELISA value (Virus titre values) ranges were recorded (Table 4). The results of DAS-ELISA showed 261 trees, out of 411 tested, infected with CTV indicating 63.50% CTV disease incidence in Assam. Results revealed presence of CTV in all the surveyed districts of Assam showing a high incidence of 78.04% CTV in Karbi Anglong district followed by 76.92% in Golaghat district (Table 5). Among the three different Citrus species, Assam lemon showed maximum incidence of CTV with 76.47% followed by Khasi mandarin (61.18%) and Gul nemu (52.03%). The results also revealed that in all the three different Citrus species Assam lemon, Gul nemu and Khasi mandarin the percentage of CTV infection was more in the higher age groups viz., > 15 years and 10 to 15 years followed by lower age group (<10 years old plants) (Table 6).
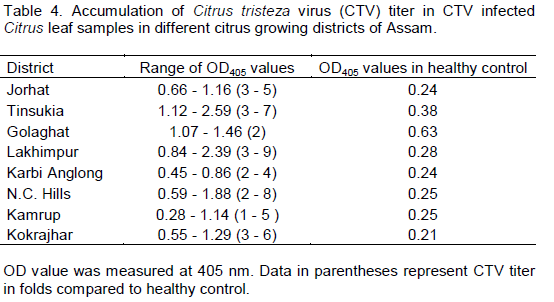
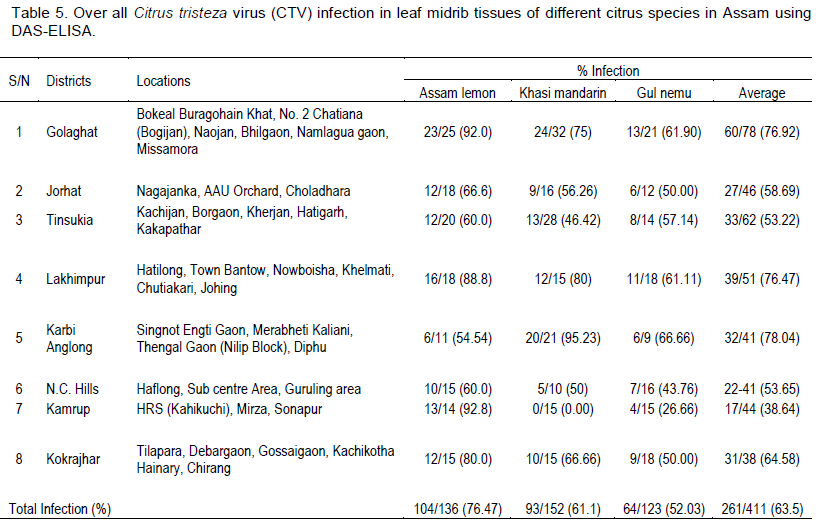
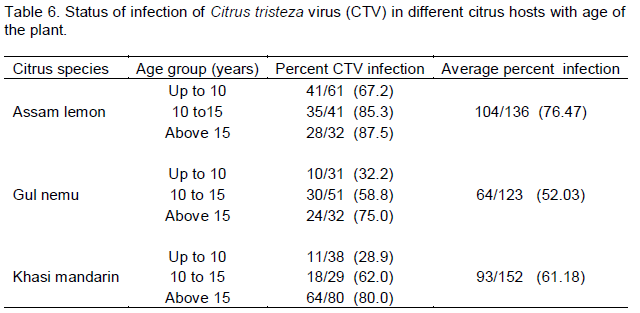
CTV infection was detected in all the surveyed locations. This is an indication that CTV is prevalent in this region. Typical symptoms of CTV infection was observed in most of the orchards and gardens. Prevalence of the vector Toxoptera spp. was noticed in all the surveyed locations. The results of DAS-ELISA showed 63.50% CTV disease incidence in Assam at different level of incidence in different survey sites. DAS-ELISA results also revealed that in all the three different Citrus species Assam lemon, Gul nemu and Khasi mandarin, the percentage of positivity is more in the higher age group. This indicates that incidence of CTV is more in matured and older plants. The results further confirm the earlier report of incidence of CTV in the citrus growing areas of Assam (Bhagabati et al., 1989). Similar type of studies were carried out by Kishore et al. (2010) who studied the assessment of CTV incidence in mandarin of Sikkim, estimated by DAS-ELISA. Biswas (2008) also diagnosed CTV in Darjeeling Hills through molecular tools and reported CTV infection up to 90.00% in mandarin trees at varying altitudes.
CTV is the most important and widely distributed disease in Assam. Laboratory based ELISA test could be successfully used to detect CTV infection in the field. Thus, findings of the research would be helpful in initiating future strategies on CTV certification, eradication, rejuvenation and management programme.
The authors declare no conflict of interest.
Authors are grateful to Dr K.K. Biswas Advanced Centre for Plant Virology, Division of Plant Pathology, Indian Agricultural Research Institute, New Delhi for internal reviewing and improvement of this paper.
REFERENCES
Azzam O, Imbe T, Ikeda R, Nath PD, Coloquio E (2001). Inheritance of resistance to rice tungro spherical virus in a near isogenic line derived from Utri Merah and in rice cultivar TKM6. Euphytica 122(1):91-97.
Crossref |
|
|
Bar-Joseph M, Garnsey SN, Gorsalves D (1979). The closteroviruses: A distinct group of elongated plant viruses. Adv. Virus Res. 25:99-168.
Crossref |
|
|
Bar-Joseph M, Marcus R, Lee RF (1989). The continuous challenge of Citrus tristeza virus control. Annu. Rev. Phytopathol. 27:291-316.
Crossref |
|
|
|
Bhagabati KN, Ahlawat YS, Chakraborty NK, Borthakur BC (1989). Distribution of greening, tristeza and mosaic disease of citrus in North Eastern States of India. Indian Phytopathol. 42:552-555. |
|
|
Biswas KK (2008). Molecular diagnosis of cirus tristeza virus in mandarin (Cirus reticulata) orchards of Darjeeling hills of West Bengal. Indian J. Virol. 19:26-31.
Crossref |
|
|
Biswas KK (2010). Molecular characterization of cirus tristeza virus isolates from the Northeastern Himalayan region of India. Arch. Virol. 155:959-63.
Crossref |
|
|
|
Cambra M, Moreno P, Navarro L (1979). Rapid detection of Citrus tristeza virus by ELISA. Anales INIA, Serie. Prot. Vegetal. 12:115-25. |
|
|
|
Capoor SP, Rao DG (1967). Tristeza irus infection of citrus in India. In : Proc. Int. Symp. or Subtrop. Trop. Hort. Bangalore pp. 723-736. |
|
|
|
Chakraborty NK, Ahlawat YS (2001). Indexing of cross protected and non-cross protected citrus cultivars/species for citrus tristeza virus by enzyme linked immunosorbent assays. Plant Dis. Res. 16:10-6. |
|
|
Clark MF, Adams AN (1977). Characteristics of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. J. General Virol. 34:475-483.
Crossref |
|
|
Edson B, Aranzazu M, Nieves C, Antonio O, de Ana L, Eduardo V, Jordi PP, Mariano C (2008). Quantitative detection of Citrus tristeza virus in plant tissues and single aphids by real-time RT-PCR. Eur. J. Plant Pathol. 120:177-88.
Crossref |
|
|
|
Ghosh SP (2007). Citrus fruits. ICAR, New Delhi. |
|
|
Karasev AV, Boyko VP, Gowda S, Nikolaeva OV, Hilf ME, Koonin EV, Niblett CL, Cline K, Gumpf DJ, Lee RF (1995). Complete sequence of the Citrus tristeza virus RNA genome. Virology 208:511-20.
Crossref |
|
|
Kishore K, Rahman H, Kalita H, Pandey B, Monika N (2010). Prevalence of Citrus tristeza virus in mandarin of Sikkim Himalayan Region. Indian J. Virol. 21:140-143.
Crossref |
|
|
|
Verma NN, Ghosh SP (1979). Citrus is North-East India. Indian Hort. 23:2-4. |