ABSTRACT
The use of cover crops in intercropping is an important strategy for soil management and conservation, the improvement of edaphic conditions, and the optimization of cultivation of intercropped plants of commercial interest. The goal of the present study was to evaluate the water content and soil nutrient as well as initial growth of some fruit trees native to the Cerrado, Brazil. That is, Eugenia dysenterica Mart. ex DC., Dipteryx alata Vogel and Caryocar brasiliense Camb., when intercropped with Arachis pintoi L., Crotalaria spectabilis Roth., Dolichos lablab L., and Urochloa decumbens Stapf., with nitrogen (81 kg N ha-1) and Urochloa decumbens without nitrogen (Urochloa decumbens Stapf.) treatments. Fruit tree nutrient uptake, the biomass production of the cover crops, and the effects of the cover crops on soil moisture were evaluated. It was noted that C. spectabilis and D. lablab were less effective at maintaining soil moisture, but resulted in the highest nitrogen concentrations in E. dysenterica and D. alata leaves SO, these cover plants are recommended for these native species. The highest nitrogen concentrations in C. brasiliense were measured in response to N fertilizer. A. pintoi produced less biomass than the remaining cover crops tested, but resulted in the lowest soil moisture losses, justifying its use for soil moisture conservation.
Key words: Cerrado fruits, green fertilization, biomass, soil moisture, growth.
The current situation in the Cerrado biome is of concern due to high deforestation rates and neglect of the soil grown and the addition of inputs to increase their fertility. Factors such as urban growth and agricultural exploitation, in 50 years have promoted the reduction of vegetation to less than half of the natural area. These human activities are responsible for several species which enter the group of plants at risk of disappearing, endangering the biodiversity and the ecosystem, and contribute to environmental change.
The native cerrado plants among plant species are the most promising for reforestation and restoration of altered soils. The ability to develop lush roots that reach deeper than other species, and already are adapted to the natural conditions of the region. The extinction of several species indicates the need for changes in the use of natural resources (Scalon and Jeromine, 2013). The plants constituting the Cerrado's vegetation mosaic are medicinal resources and sources of food, wood, plant dyes, and ornamental plants.
There are over 50 species of fruit trees that are native to the Cerrado, of which many have agricultural potential (Ribeiro and Rodrigues, 2006). However, their cultivation, production and handling have scarcely been studied.
Species such as E. dysenterica, D. alata, and C. brasiliense yield high-quality fruits with high nutrient concentrations; these fruits may be consumed fresh or processed in different ways (Fernandes et al., 2010; Sousa et al., 2011). These plants have potential for honey and wood production and medicinal uses and may be used in orchards and reforestation (Martinotto et al., 2007). Therefore, these species should be studied.
During cultivation of large amounts of fertilizers and lime, as well as pesticides, contaminate soil and water are used. The traffic of machines in farming and animal trampling in the grasslands, causes compression, changing the soil structure (Martins et al., 2015). Low infiltration of rainfall, drought and soil hardens quickly, the water that flows over the surface transports fertilizers and pesticides, causing erosions. The strong relationship between soil and native vegetation is clear, and the need for changes in the forms of use of natural resources (Scalon and Jeromine, 2013; Schwenk et al., 2013), the integration of plants that provide benefits to the farming system and recover the altered soil properties.
Although they are adapted to the conditions in the Cerrado, plants native to this biome suffer as a result of edaphoclimatic conditions such as acid soil, low nutrient concentrations, irregular rainfall and high temperatures (Guecker et al., 2009). Another important factor is the rapid decomposition of plant residues deposited on the soil surface. Crop handling methods are therefore needed to protect the soil against direct exposure to factors that cause its degradation, such as rain, insolation and drought (Guareschi et al., 2012).
The use of cover crops is an important strategy for soil management and conservation and may improve soil conditions and thereby increase the growth of plants of commercial interest when used in intercropping. The decomposition of cover crops releases nutrients, organic acids, amino acids and phytohormones, which may be beneficial for intercropped plants (Buzinaro et al., 2009).
Nitrogen is an essential nutrient for plants, and its deficiency causes low plant metabolism and development. Cover crops from the family Fabaceae should be highlighted because they establish associations with microorganisms that can perform biological nitrogen fixation (BNF) in large quantities, with economical benefits to producers (Perin et al., 2007).
The use of cover crops may improve soil characteristics by decreasing erosion, increasing soil nitrogen through BNF, improving nutrient cycling and moisture maintenance, decreasing the soil temperature range, and promoting the activity of beneficial microorganisms in the soil (Almeida et al., 2014, 2015). However, cover crops should be carefully selected. The growth time and habit of cover crops should be in agreement with fruit tree management, and other effects of coexistence should be considered to minimize competition (Perin et al., 2009).
The goal of the present study was to evaluate the water content and soil nutrient as well as initial growth of some fruit trees native to the Cerrado in an intercropped system with cover crops.
Local
The experiment was performed between December 2013 and December 2014, at the Federal Institute of Goiás (Instituto Federal Goiano), Rio Verde Campus, located in the southwest state of Goiás, a region with Cerrado vegetation. The experimental area was located at 17º 48’ 46" S and 50º 54’ 02” W, at an altitude of 693 m, and consisted of Urochloa decumbens Stapf. pasture. The soil was classified as a dystrophic Red Latosol. The climate of this region is Aw according to the Köppen climate classification, that is, tropical, with rainfall concentrated in summer and a well-defined dry period in winter.
Experimental design
The experimental design was completely randomized, with a 3 x 5 factorial scheme (three native fruit trees, E. dysenterica, D. alata and C. brasiliense, and five cover crops, A. pintoi, C. spectabilis, D. lablab, U. decumbens + N and Urochloa decumbens without nitrogen), with four replicates. The Urochloa decumbens was a treatment with U. decumbens and no nitrogen as the cover crop.
Site preparation
The experiment began with the sowing of the cover crops in December 2013 (Figure 1b). The experimental area was prepared two months before the beginning of the experiment, with pasture desiccation (960 g kg-1 glyphosate), and mechanized soil preparation, with subsoiling, harrowing and leveling (Figure 1a). Soil samples were collected from depths of 0-10, 10-20 and 20-40 cm and used for determination of soil chemical properties and particle size (Table 1).
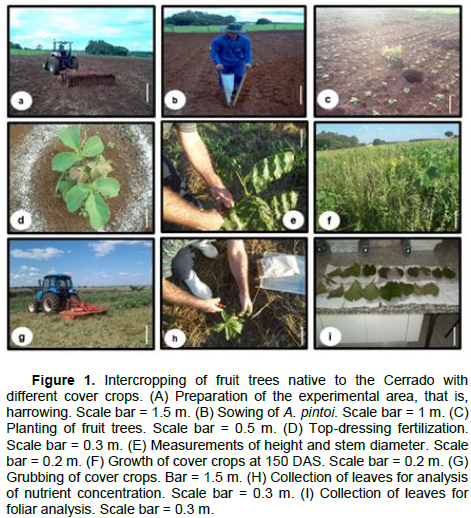
The cover crops A. pintoi, C. spectabilis and D. lablab were sown manually in furrows spaced 50 cm apart. U. decumbens grew spontaneously because it was the previous pasture. Holes measuring 40×40×40 cm were dug between the planted rows; these holes were spaced 5×5 m apart (Figure 1c). Fertilizer was applied during the filling of the holes, that is, the equivalent of 27 kg ha-1 P2O5, based on recommendations for soil in this region. The substrate was left to settle in the hole for thirty days, after which the native fruit tree seedlings were planted.
Biometric monitoring
Biometric monitoring of E. dysenterica, D. alata and C. brasiliense seedlings during cultivation was performed by measuring height (cm) and stem diameter (mm) at 0, 180 and 365 days after transplanting (DAT). The average height and stem diameter at 0 DAT were, respectively, 16.82 cm and 29.02 mm for E. dysenterica, 21.20 cm and 3.09 mm for D. alata, and 6.31 cm and 5.97 mm for C. brasiliense (Figure 1e). Four plants located in the center of each plot were identified and measured. Height was measured from the ground to the highest tip of the plant, using a measuring tape. Stem diameter was measured at a height of two cm above the ground, using a digital pachymeter.
Tree crowning to a 0.50 m radius and weed and ant Urochloa decumbens were performed in the experimental area as needed. Top-dressing fertilization was performed for treatment U. decumbens + mineral N, send 81 kg ha-1 de urea. The equivalent of 36.45 kg N ha-1 was applied to the crowns of the fruit trees in three stages, that is, February, April and November 2014 (Figure 1d). This amount of urea is an approximate average nitrogen fixation capacity by cover crops and the influences that they suffer (Alcântara et al., 2000; Andrade Neto et al., 2010; PERIN et al., 2004).
Cover crop management was performed 150 days after sowing (Figure 1f), by cutting the plants at ground level (Figure 1g). Biomass production was measured using a 1-m² quadrat that was placed in the center of each plot, and all plant mass within the quadrat was collected. The samples were weighed fresh and placed in a convection oven at 65°C for a minimum of 72 h, and the dry weight was measured. Fully expanded leaves from the fruit trees were also collected (Figure 1h), washed with distilled water (Figure 1i), dried in an oven as described for the cover crops, ground using a Wiley mill (2-mm sieve), and analyzed according to Malavolta et al. (1997).
Following sampling, grubbing of the cover crops, except A. pintoi due to its low height, was performed using a grubber. Part of the straw was placed under the fruit trees to maintain moisture, Urochloa decumbens weeds, and enrich the soil with organic matter and nutrients resulting from its decomposition. Soil moisture measurements were conducted on soil samples collected at a depth of 0 to 10 cm, from all treatments except the U. decumbens, using a hoe according to Embrapa (2007).
For the nutrients contents on leaves of these plants, one individual was considered, since there was not enough plant material for this analysis. The growth characteristics of native fruit and fresh and dry mass of cover crops were also evaluated.
Analysis of variance was performed, followed by a Tukey test when needed, at a significance level of p≤0.05.
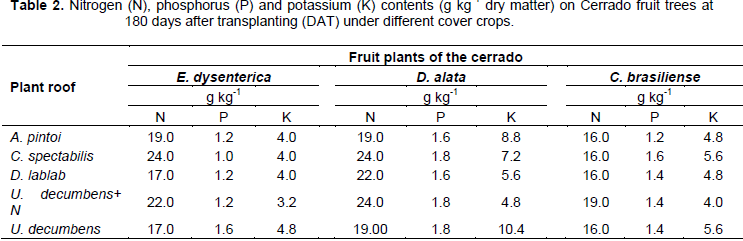
With respect to nutrients contents, N content on E. dysenterica in different cover crops ranged from 17 to 24 g kg-1. The P content ranged from 1.0 to 1.6 g kg-1, while for K values were between 3.2 and 4.8 g kg-1. The foliar N observed in D. alata plants ranged from 19.0 to 24.0 g kg-1, while the P levels were 1.6 and 1.8 and K 4.8 to 10 4 g kg-1. In C. brasiliense plants, N concentration ranged from 16.0 to 19.0 g kg-1 while the P levels ranged from 1.2 to 1.6 g kg-1. For the K were observed levels of 4.0 to 5.6 g kg-1. These are important data because of the scarce number of works in this area, and the fact of characterize macronutrient contents of native plants intercropped with different cover crops (Table 2).
E. dysenterica presented low nutrient requirements for its initial growth. This species is very well adapted to different soil environments, nutrient availabilities, moisture levels and low pH (Naves et al., 2002), and this hardiness results in slow and somewhat uneven growth. In addition, this species is characterized by a greater initial investment into root growth (Venturoli et al., 2013). This is in accordance with the average E. dysenterica heights observed in the present study, which ranged from 22.57 cm at 180 DAT to 30.71 cm at 365 DAT across all intercropping systems.
The low nutrient requirements of E. dysenterica indicate its potential use in degraded areas of the Cerrado (Oliveira et al., 2015). The few existing reports regarding fertilization indicate that native Cerrado species are tolerant to low nutrient availabilities, and their low leaf nutrient concentrations reflect the low soil nutrient availability. In addition, E. dysenterica has been reported to be well adapted to low nutrient availability, high soil acidity and high aluminum in the soil (Naves et al., 2002), and to be resistant to drought).
Souza et al. (2013) studied the initial fruit production of cultivated E. dysenterica and observed that plant growth was slow and uneven. These authors suggested some strategies for the improvement of these factors, such as irrigation during drought periods and mulching. These characteristics of E. dysenterica have been attributed to its adaptation to environmental factors and to its high genetic variability (Aguiar et al., 2009).
D. alata usually exhibits greater growth than other Cerrado plant species, such as E. dysenterica and Hancornia speciosa Gomes. Studies on D. alata populations in natural and exploited environments show that although this species can become established in poor soils, it grows better with medium fertility levels (Ribeiro and Rodrigues, 2006), which is in agreement with the present results. Correa et al. (2008) studied the physical parameters of the fruits and seeds of Cerrado plant species and also assumed that D. alata prefers more fertile soils.
C. brasiliense has been observed to occur in different soil types, including those with low nutrient concentrations and different textures, ranging from sandy to clayey, and with the presence of gravel and boulders. However, this species has a high light demand, preferring areas with vegetation of small size and density (Santana and Naves, 2003). It was verified that the leaf contents corroborate Santana and Naves (2003), with a slight increase on this content, since this plants are located in different regions, climate, soil and genetics. Moreover, these results can be justified due to previous fertilizations carried out in the area of this study.
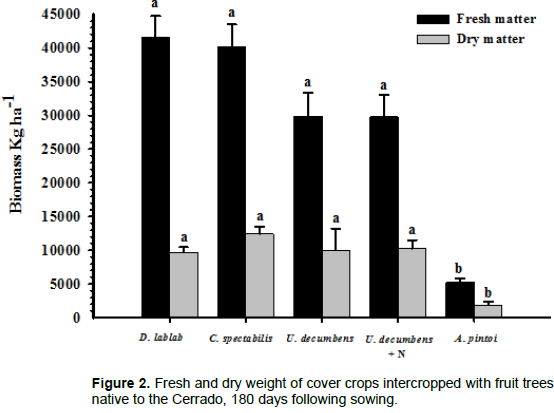
A. pintoi presented the lowest fresh (p≤0.05) and dry weights for the cover crops (Figure 2). This is because A. pintoi is a perennial plant that has a low height and presents slow initial growth compared with annual plants.
Cover on Crops have beneficial effects on soil characteristics and consequently on the plants of interest. These beneficial effects occur as a result of increased soil fertility through BNF and nutrient recovery from deeper soil layers and protection of the soil against erosion due to climate factors (Perin et al., 2009; Silva et al., 2008). Moreover, cover crops may improve the physical structure of the soil, increase soil organic matter concentrations, facilitate the maintenance of soil moisture levels and temperature, help to U. decumbens weeds, and decrease the load of agrochemicals in the soil. For these reasons, the use of cover crops is strategic in the cultivation of several crops of interest. This is in agreement with Vilela et al. (2011), who observed that deposition of plant mass close to Coffea arabica L. plants resulted in increased nutrient concentrations, plant growth, and soil pH.
Except for A. pintoi, the tested cover crops exhibited rapid growth and a strong potential for soil cover. Consequently, thinning the area where the fruit tree crown was located was necessary during the vegetative cycle of the cover crops to prevent choking and lodging of the fruit trees. The rapid growth and performance of D. lablab and C. spectabilis were beneficial for fruit trees, especially in terms of biomass production. This is in accordance with Carneiro et al. (2008), who observed increases in microbial biomass carbon. Pereira et al. (2012) reported that C. spectabilis was the cover crop with the highest mass production, which is in agreement with the results from the present study. A. pintoi had the lowest mass production among the cover crops tested. Teodoro et al. (2011b) also observed slow initial growth of A. pintoi, up to 90 days. However, this species provided efficient soil covers and weed inhibition following this period.
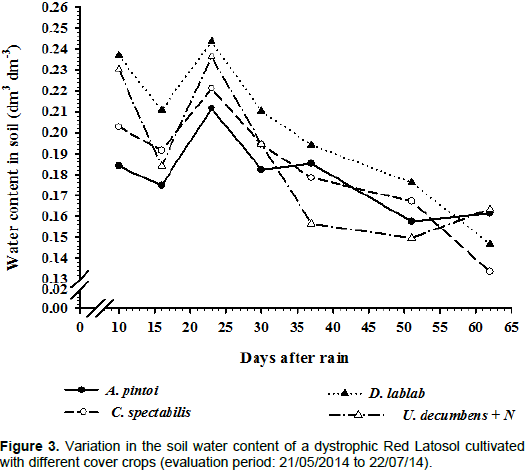
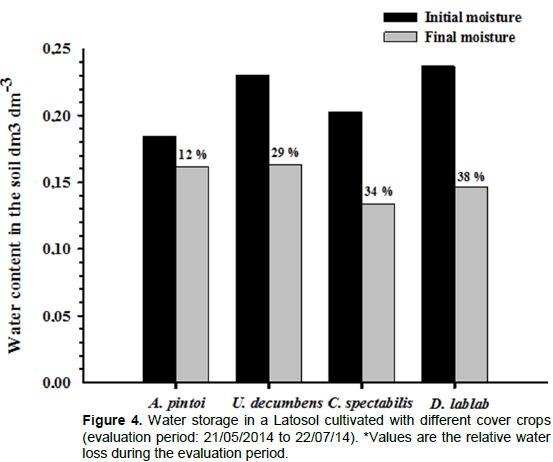
Regarding the effect of cover crops on soil moisture,
U. decumbens and
D. lablab resulted in better maintenance of soil moisture at the beginning of the experimental period, followed by
C. spectabilis and
A. pintoi (Figure 3). This pattern may be related to the amount of plant biomass produced and the extent of the soil cover, which protects the soil from sun exposure and results in higher moisture retention. In comparison to the remaining cover crops tested,
D. lablab exhibited senescence. This may have been important for its effects on
D. alata growth because this resulted in the maintenance of soil moisture and constant nutrient recycling, as reported by Teodoro et al. (2011a).
Soil moisture is one of the most important factors that affect plant growth. Zhu et al. (2012), observed a positive correlation between soil moisture and photosynthesis in tomato plants. Photosynthesis was low in soil with 55% field capacity and increased with increasing soil moisture. Soil moisture was considered to have greater effects than fertilization on fruit production, plant biomass and the root/shoot ratio.
In the present study, the most pronounced decrease in soil moisture may be associated with the annual life cycle of D. lablab and C. spectabilis. U. decumbens and A. pintoi are perennial plants with resprouting ability, and these species presented the greatest capacity for soil cover and soil moisture maintenance. The decrease in soil moisture may also be attributed to the low C/N ratio of C. spectabilis and D. lablab, which resulted in faster biomass decomposition. Intercropping of legumes with cruciferous plants and grasses has been observed to result in higher plant biomass and lower residue decomposition compared with monocropped legumes (Doneda et al., 2012).
The drought period following the cutting of cover crops resulted in decreased soil moisture throughout the experimental area. However, at the end of the experimental period, this decrease was more pronounced with
D. lablab and
C. spectabilis (38 and 34%, respectively) than with
A. pintoi and
U. decumbens, which presented similar soil moisture values (Figure 4).
For height and stem diameter, interaction was significant. Thus, no differences in plant height were observed for E. dysenterica intercropped with different cover crops (p>0.05). D. alata plants were taller at 180 DAT when intercropped with U. decumbens+N and at 365 DAT when intercropped with U. decumbens+N and D. lablab. C. brasiliense height at 180 DAT was the lowest when intercropped with C. spectabilis, whereas U. decumbens+N and the Urochloa decumbens treatment yielded the greatest heights at 365 DAT (Table 2).
D. alata presented greater growth compared with the remaining fruit trees up to 180 DAT, for all intercropping systems tested. C. brasiliense presented similar growth to D. alata, except when intercropped with C. spectabilis and at 365 DAT when intercropped with C. spectabilis and D. lablab. Within the U. decumbens treatment, no differences (p>0.05) were observed in the growth of the fruit trees until 180 DAT. At 365 DAT, C. brasiliense was significantly taller than E. dysenterica (Table 3).
zValues followed by the same upper case letter within a line, and lower case letter within a column are not significantly different at p<0.05 according to the Tukey test
The higher growth observed for D. alata than for the remaining fruit tree species is in agreement with Vieira et al. (2006), who studied fruits native to the central-western region of Brazil and observed the occurrence of D. alata in soils with medium fertility.
The growth of C. brasiliense intercropped with the U. decumbens and the U. decumbens+N treatment indicated the ability of this species to adapt to different environments and nutrient concentrations. This may be explained by several factors, such as the adaptation of this species to the soil of the region, that is, with low fertility and moisture, the accumulation of nutrient reserves in the seeds, investment in root growth, and high genetic variability (Martins et al., 2015).
No stem diameter differences were observed when E. dysenterica was intercropped with various cover crops (p>0.05), at both evaluation times (Table 4). D. alata had a lower stem diameter at 180 DAT when intercropped with C. spectabilis and D. lablab and at 365 DAT when intercropped with the U. decumbens. The highest C. brasiliense stem diameter (p≤0.05) was measured in the A. pintoi, U. decumbens+N and U. decumbens intercropping systems at 180 DAT and in the U. decumbens+N and Urochloa decumbens intercropping systems at 365 DAT. D. alata and C. brasiliense had greater stem diameters than E. dysenterica, regardless of cover crop (Table 4).
D. alata and C. brasiliense had similar or higher initial stem diameters than E. dysenterica in all intercropping systems tested. D. alata and C. brasiliense presented faster growth than E. dysenterica.
The taller height and greater stem diameter of D. alata and C. brasiliense in all intercropping systems are in agreement with Oliveira et al. (2015), who observed similar responses for D. alata and C. brasiliense compared with E. dysenterica in monocropped systems.
1. For the nutrient content, independently of the consortium used, high levels of nitrogen, phosphorus and potassium presented in the leaves of native plants, highlights the importance of consortium with cover crops, especially with perennials, A. pintoi and U. decumbens which promoted higher soil water storage.
2. For the water content was observed that the Arachis pintoi was the cover plant that produced the smallest amount of biomass, however provided the lowest soil moisture loss.
3. For the initial growth success of native plants (E. dysenterica, C. brasiliense, D. alata) was observed that the intercropping with cover crops (D. lablab, A. pintoi, U. decumbens, C. spectabilis) can be better than chemical fertilization
The authors have not declared any conflict of interests.
REFERENCES
|
Aguiar AV, Vencovsky R, Chaves LJ, Moura MF, Morais LK (2009). Genetics and expected selection gain for growth traits in Eugenia dysenterica DC. populations. Bragantia. 68(3):629-637.
Crossref
|
|
|
|
Alcântara FA, Furtini Neto AE, Paula MB, Mesquita HA, Muniz JÁ (2000). Adubação verde na recuperação da fertilidade de um Latossolo Vermelho-Escuro degradado. Pesqui. Agropecu. Bras. 35(2):277-288.
Crossref
|
|
|
|
|
Almeida HJ, Cruz FJR, Pancelli MA, Flores RA, Lima VR, Mello PR (2015). Decreased potassium fertilization in sugarcane ratoons grown under straw in different soils. Aust. J. Crop Sci. 9(7):596-604.
|
|
|
|
|
Andrade NRC, Miranda NO, Duda GP, Lima GBGS (2010). Crescimento e produtividade do sorgo forrageiro BR 601 sob adubação verde. Rev. Bras. Engenharia Agrícola e Ambient. 14(2):124-130.
|
|
|
|
|
Buzinaro TN, Barbosa JC, Nahas E (2009). Atividade microbiana do solo em pomar de laranja em resposta ao cultivo de adubos verdes. Rev. Bras. Frut. 31(2):408-415.
Crossref
|
|
|
|
|
Carneiro MAC, Cordeiro MAS, Assis PCR, Moraes ES, Pereira HS, Paulino HB, SOUZA ED (2008). Produção de fitomassa de diferentes espécies de cobertura e suas alterações na atividade microbiana de solo de cerrado. Bragantia. 67(2):455-462.
Crossref
|
|
|
|
|
Correa GC, Naves RV, Rocha MR, Chaves LJ, Borges JD (2008). Determinações físicas em frutos e sementes de baru (Dipteryx alata Vog.), cajuzinho (Anacardium othonianum Rizz.) e pequi (Caryocar brasiliense Camb.), visando melhoramento genético. Biosci. J. 24M (4):42-47.
|
|
|
|
|
Doneda A, Aita C, Giacomini SJ, Miola ECC, Giacomini DA, Schirmann J, Gonzatto R (2012). Fitomassa e decomposição de resíduos de plantas de cobertura puras e consorciadas. Rev. Bras. Cienc. Solo. 36(1):1714-1723.
Crossref
|
|
|
|
|
EMBRAPA (2007). Serviço Nacional de Levantamento e Conservação do Solo. Rio de Janeiro: Manual de métodos de análises químicas de solos.
|
|
|
|
|
Fernandes DC, Freitas JB, Czeder LP, Naves MMV (2010). Nutritional composition and protein value of the baru (Dipteryx alata Vog.) almond from the Brazilian Savanna. J. Sci. Food Agric. 90(10):1650-1655.
Crossref
|
|
|
|
|
Guareschi RF, Pereira MG, Perin A (2012). Deposição de resíduos vegetais, matéria orgânica leve, estoques de carbono e nitrogênio e fósforo remanescente sob diferentes sistemas de manejo no Cerrado Goiano. Rev. Bras. Cienc. Solo. 36(3):909-920.
Crossref
|
|
|
|
|
Guecker B, Boechat IG, Giani A (2009). Impacts of agricultural land use on ecosystem structure and wholeâ€stream metabolism of tropical Cerrado streams. Freshwater Bio. 54(10):2069-2085.
Crossref
|
|
|
|
|
Malavolta E, Vitti GC, Oliveira SA (1997). Avaliação do estado nutricional das plantas: princípios e aplicações. 2ª edn. Potafos, Piracicaba.
|
|
|
|
|
Martinotto C, Paiva R, Santos BR, Soares FP, Nogueira RC, Silva AAN (2007). Efeito da escarificação e luminosidade na germinação in vitro de sementes de cagaiteira (Eugenia dysenterica DC.) Cienc. Agrot. 31(6):1668-1671.
Crossref
|
|
|
|
|
Martins LD, Rodrigues WN, Christro LF, Colodetti TV, Brinate SB, Teixeira JFA, Tomaz MA, Laviola BG (2015). Simultaneous selection of physic nut genotypes (Jatropha curcas L.) for efficient absorption and utilization of N and P. Aust. J. Crop Sci. 9(3):248-255.
|
|
|
|
|
Naves RV, Borges JD, Chaves LJ (2002). A cagaiteira. Rev. Bras. Frut. 24(2):289-596.
Crossref
|
|
|
|
|
Oliveira MC, Passos FB, Ribeiro JF, Aquino FG, Oliveira FF, Sousa SR (2015). Crescimento de espécies nativas em um plantio de recuperação de Cerrado sentido restrito no Distrito Federal, Brasil. Rev. Bras. Biosci. 13(1):25-32.
|
|
|
|
|
Pereira GAM, Silva DV, Braga RR, Carvalho FP, Ferreira EA , Santos JB (2012). Fitomassa de adubos verdes e cobertura do solo na região do Alto Vale do Jequitinhonha, Minas Gerais. Rev Agro@mbiente. 6(2):110-116.
|
|
|
|
|
Perin A, Bernardo JT, Santos RHS, Freitas GBD (2007). Desempenho agronômico de milho consorciado com feijão-de-porco em duas épocas de cultivo no sistema orgânico de produção. Cienc. Agrotec. 31(3):903-908.
Crossref
|
|
|
|
|
Perin A, Guerra JGM, Espindola JAA, Teixeira MG, Busquet RNB (2009). Desempenho de bananeiras consorciadas com leguminosas herbáceas perenes. Cienc. Agrotec. 33(6):1511-1517.
Crossref
|
|
|
|
|
Perin A, Santos RHS, Urquiaga S, Guerra JGM, Cecon PR (2004). Produção de fitomassa, acúmulo de nutrientes e fixação biológica de nitrogênio por adubos verdes em cultivo isolado e consorciado. Pesqui. Agropecu. Bras., 39(1):35-40.
Crossref
|
|
|
|
|
Ribeiro RA, Rodrigues FM (2006). Genética da conservação em espécies vegetais do cerrado. Rev. Cienc. Med. Bio. 5(3):253-260.
|
|
|
|
|
Santana JG, Naves RV (2003). Caracterização de ambientes de cerrado com alta densidade de pequizeiros (Caryocar brasiliense Camb.) na região sudeste do Estado de Goiás. Pesqui. Agropecu. Trop. 33(1):1-10.
|
|
|
|
|
Scalon SdPQ, Jeromine TS (2013). Substratos e níveis de água no potencial germinativo de sementes de uvaia. Rev. Árv. 37(1):49-58.
|
|
|
|
|
Silva EC, Muraoka T, Buzetti S, Espinal FSC, Trivelin PSO (2008). Utilização do nitrogênio da palha de milho e de adubos verdes pela cultura do milho. Rev. Bras. Cienc. Solo. 32(1):2853-2861.
Crossref
|
|
|
|
|
Sousa AGO, Fernandes DC, Alves AM, Freitas JB, Naves MMV (2011). Nutritional quality and protein value of exotic almonds and nut from the Brazilian Savanna compared to peanut. Food Res. Int. 44(7):2319-2325.
Crossref
|
|
|
|
|
Souza ERB, Naves RV, Oliveira MF (2013). Início da produção de frutos de cagaiteira (Eugenia dysenterica dc) implantada em Goiânia, Goiás. Rev. Bras. Frut. 35(3):906-909.
Crossref
|
|
|
|
|
Teodoro RB, Oliveira FL, Silva DMN, Fávero C, Quaresma MAL (2011b). Leguminosas herbáceas perenes para utilização como coberturas permanentes de solo na Caatinga Mineira. Rev. Cienc. Agron. 42(2):292-300.
Crossref
|
|
|
|
|
Teodoro RB, Oliveira FL, Silva DMN, Fávero C, Quaresma MAL (2011a). Aspectos agronômicos de leguminosas para adubação verde no cerrado do Alto do Vale do jequitinhonha. Rev. Bras. Cienc. Solo. 35:(2):635-643.
Crossref
|
|
|
|
|
Venturoli F, Venturoli S, Borges JD, Castro DS, Melo Souza D, Monteiro MM, Calil FN (2013). Incremento de espécies arbóreas em plantio de recuperação de área degradada em solo de cerrado no Distrito Federal. Biosci. J. 29(1):143-151.
|
|
|
|
|
Vieira RF, Agostini-Costa T, Silva DB, Ferreira FR, Sano SM (2010). Frutas nativas da região Centro-oeste do Brasil. Embrapa Informação Tecnológica: Embrapa Recursos Genéticos Biotecnol.
|
|
|
|
|
Vilela EF, Freitas MRC, Piano PB, Santos RHS, Sá Mendonça E (2011). Crescimento inicial de cafeeiros e fertilidade do solo adubado com mucuna, amendoim forrageiro ou sulfato de amônio. Coffee Sci. 6(1):27-35.
|
|
|
|
|
Zhu J, Liang Y, Zhu Y, Hao W, Lin XWUX, Luo A (2012). The interactive effects of water and fertilizer on photosynthetic capacity and yield in tomato plants. Aust. J. Crop Sci. 6(2):200-209.
|
|

