ABSTRACT
For elucidating the genetic basis of N, P and K contents in kernels and stalks in maize under different nitrogen supply condition, a set of 203 F2: 4 / F2: 5 family lines, derived from an elite maize hybrid Nongda108, were tested under nitrogen plus (N+) and no nitrogen plus (N-) treatments in the field over two years, and a genetic linkage map was constructed with 199 SSR molecular markers, covered 2100.9 cm for 10 chromosomes with an average interval length of 10.82 cm. The results showed that low N stress not only affected N content in maize kernels and stalks, but also affected the absorption and transportation of P and K contents in some degree. A total of 34 quantitative trait locus (QTL) including 15 QTLs in kernels and 19 QTLs in stalks for N, P, K content were identified by means of the composite interval mapping method (CIM), of which, 13, 9 and 12 QTLs detected for N, P, K content, respectively. Each QTL could explain the variance of phenotype ranged in turn from 7.30 to 31.09%, 7.57 to 14.3% and 8.11 to 32.82% for three main mineral elements content. The QTL qNC4c, qPC9b, qKC10b as well as qNC4b, qPC5b, qKC6a were main contributing QTL for N, P, K contents in kernels and stalks. Out of these QTLs detected for N, P, K contents in kernels and stalks, the results also implied that the loci derived from Huang C played important roles in N, P, K absorption, while the loci from Xu178 played marked roles in N, P, K transportation from stalks to grains.
Key words: Maize, low nitrogen stress, N, P, K content, quantitative trait locus (QTL), analysis.
Nitrogen (N) fertilizer plays important roles in increasing yield and improving quality in maize production. The previous studies have demonstrated that the biomass, grain yield as well as nutrient quality for foodstuff in maize are higher under high N condition than low N condition (Agrama et al., 1999; Zhu and Chen, 2002; Aildson et al., 2005; Martín et al., 2008). Presterl et al. (2003) reported that about 37% grain yield was lost under N- condition compared with N+ condition, and the agricultural and yield related traits among F2:3 families had significant genetic difference under high N (280 kg.ha-1) and low N (30 kg.ha-1) conditions (Agrama et al., 1999). For getting a high grain yield in maize production, the amount of nitrogen fertilizer input has increased gradually in recent years, especially in some countries or areas, the amount of nitrogen fertilizer input has far excess the real need of maize development, leading to the nitrogen using efficiency (NUE) decrease, induced the underground water pollution because of nitrate prediction, and increased the cost of agricultural production (Machado et al., 1992; Zhang et al., 1995), such as in China, the NUE is only 30 to 40% in some season, so grain yield in maize would be increased with adding nitrogen application within a certain range (Pan et al., 1995; Raja 2001). For getting a high grain yield, the commercial hybrids with NUE are required in maize production, so in maize breeding, it is urgent affairs of how to increase NUE in maize, and it is also the necessity requirement in the development of zoological agriculture (Zhang et al., 1995; Gallais and Hirel, 2004).
Many reports have showed significant differences existing in nitrogen absorption, transportation and utilization in some genotypes of maize, and some greater progress have gained by predecessors on the basis of physiology, biochemistry, phenotypes, germplasm collection and improvement (Machado et al., 1992; Zhang et al., 1997; Hirel et al., 2001; Presterl et al., 2003). For disserting the genetic bases of nitrogen usage, Bertin and Gallias (2001) had detected the QTLs for grain yield, nutrient components in maize kernels under N+ and N- conditions using a set of recombinant inbred lines (RIL) and its test population, they found that more QTLs were detected under N+ condition than N- condition. Gallais and Hirel (2004) reported that genotype × nitrogen interaction was significant for grain yield and explained by variation in kernel number. Also, in N- condition, N-uptake, the nitrogen nutrition index, and glutamine synthetase activity in the vegetative stage were positively correlated with grain yield, whereas leaf senescence was negatively correlated. The gene of glutamine synthetase locus on chromosome 5 in maize appears to be a good candidate, which can, at least partially, explain the variation in NUE (Gallais and Hirel, 2004). While in maize, more trials indicated that the NUE varied significantly with genotypes (Hirel et al., 2001; Presterl et al., 2003; Monneveux et al., 2005).
Oikeh et al. (1998) even trailed with five maize cultivars under four N levels (0, 30, 60, and 120 kg ha-1) and showed that increasing N levels increased grain yield, kernel weight, and grain protein greatly for all the cultivars. While N is a component of protein, so it inferred that lack of nitrogen might reduce grain N content greatly. The similar results also reported by Liu et al. (2008) in maize, they showed that the grain yield, grain protein and oil contents in F2:3/F2:4 populations were significantly reduced, but the starch a little increased under N- conditions compared with N+ conditions at two locations.
Nitrogen is one of the three essential elements in nutrition for maize, low N stress not only affects corn growth, also leads to lose balance among nutrient elements. Teng et al. (2005) has made a trial on the effect of soybean yield under different ratios of N, P and K, their results showed that when the P and K fertilizer were enough, the soybean yield was even less under over nitrogen supplied than no N supply, while more yield could be gained with a suitable N supply. It implied that a proper ratio of N, P and K application was needed to get a reasonable high yield. Aildson et al. (2005) reported that N nutrient application could increase grain yield and grain N concentration in maize, while improved the kernel quality such as reducing breakage susceptibility. Although the nitrogen supply can affect the P, K absorption as well as the distribution in maize kernels and stalks; however there have few reports on the effect of low nitrogen stress to the contents of nitrogen (N), phosphorous (P) and potassium (K) as well as the corresponding QTL analysis in maize kernels and stalks.
The aims of this study were to (i) investigate the variance of N, P, K content in maize kernels and stalks by using a set of F2:4 and F2:5 family lines under two nitrogen conditions, (ii) identify the QTL for N, P, K contents in maize kernels and stalks under N+ and N- conditions, and (iii) finally expect to fulfill the theory foundation for further carrying out the research on high using efficiency of essential nutrients in maize.
Materials supply and soil conditions
A set of 203 F2:3 / F2:4 population derived from an elite maize hybrid, Nongda 108 (Huang C x Xu178), was used as a basic material in the study. The hybrid has extended widely in China, for its two parents, the inbred line Xu178 has high NUE, and Huang C was sensitive to nitrogen element (Chen et al., 2003; Tang et al., 2005a). The leaf DNA of F2 individuals was extracted by modified SDS method (Saghai et al., 1984). Because the kernels in the ears of F2:3/F2:4 families were F2:4/ F2:5 progeny respectively, therefore, the content of N, P, K in kernels of the F2:3/F2: 4 ears was described in this paper as the F2:4/ F2:5 population which was one more generation than stalks in turn.
The soil nutrient composition in 0-20 cm top layer was tested according to the method by Bao (2000), the contents of N, P, K in the soil were tested by the method of Kjedahl’s for N test, colorimetry for P and blaze luminosity for K, respectively. The soil condition was 8.52 g.kg-1 organic materials, 0.78 g.kg-1 total nitrogen, 8.6 mg.kg-1 available phosphorous, 69.2 mg.kg-1 available potassium. The soil was lack of nitrogen based on the plentiful or lack index in North China.
Field evaluation and data analysis
The F2:3 / F2:4 populations were planted in Xinzheng Agricultural Institute (Xinzheng, China) in 2004 and 2005, which located at the main maize belt in China. The experimental design in the field was under a split block design with two replications, each material was planted in one plot, with the length of 4 m and 0.67 m apart between two rows, each row with 15 plants and the total density was 56250 plants per hectare. Nitrogen was the main treatment including nitrogen supply (N+) and no nitrogen supply (N-), and the segregation family was subsidiary treatments. At seedling stage, 67.5 kg.hm-2 P2O5 (calcium superphosphate) and 101.3 kg.hm-2 k2O(potassium nitrate) were fertilized to all N+ and N- treatments, and 175kg.hm-2 pure N was supplied only to N+ treatment, other managements and irrigation just the same as common field cultivation.
The ear of five consecutive plants for each material was bagged before silking, and self-pollinated twice by hand in the field after the all silk was out. After mature, plants were harvested including ears and stalks, and air-dried respectively, then the stalks from one plot were mix smashed by miller with 60 eyes screen. The contents of N, P, K in kernels and stalks of each 3 samples from each plot were also tested according to the method by Bao (2000). The basic data of the three nutrients content in the population over two years were analyzed by SPSS software, respectively, and the broad-sense heritability of measured trait was computed according to Knapp et al. (1985) method.
Genetic linkage map construction and QTL mapping
In total, 635 pairs of simple sequence repeat (SSR) markers were selected from the maize genome database (http://www.maizegdb.org) to screen for polymorphism between the two parents, Huang-C and Xu178. Of these, 235 SSR markers displayed distinct polymorphism and were used to amplify the DNA of the individuals in the F2 population. Molecular linkage maps were constructed with MAPMAKER 3.0 (Lander et al., 1987) at a logarithm of odds (LOD) threshold = 3.0.
The composite interval mapping method (Zeng, 1994) and Model 6 of the Zmapqtl module of QTL Cartographer 2.5 (North Carolina, USA) were used to identify QTL (Wang et al., 2004). The threshold of a LOD was calculated using 1000 times permutation at a significance level of P = 0.05, with scanning intervals of 2 cm between markers and putative QTL and with a 10 cm window (Churchill and Doerge, 1994; Doerge and Churchill, 1996). The number of marker cofactors for background control was set by forward–backward stepwise regression with five controlling markers. Two putative QTL, detected in different nitrogen treatments or locations within 10 cm, were considered as the same QTL (Stuber et al., 1987).
The N, P, K contents in maize kernels under two nitrogen conditions
The mean content of N, P, K in the kernels of F1, parents and the F2?4 / F2?5 populations showed some differences between the two N levels in 2004 and 2005 (Table 1). The N content in the kernels of F2?4 family lines was significantly increase under N+ condition comparing with N- condition (t= 3.36; t0.05= 1.96; t0.01= 2.58), while P and K content decreased obviously (t= -3.17 and t= -4.95). The N content in the kernels of F2?5 family lines was also significantly increased (t= 5.74), but no significant differences were found in P and K content (t=-1.39 and t= 0.73). It inferred that adding N input was benefit to increase the N but P and K in maize grain. The increasing range of N content of hybrid Nongda108 was lower than that of the parent Huang C yet. The N content in the kernels of Huang C was decreased by 18.13 and 15.90% under N- condition compared with N+ condition in 2004 and 2005, respectively; 8.26 and 6.62% decreased in F1 while 4.83 and 8.63% increased in parent Xu178. The broad sense heritability (HB2) of N, P, K contents in maize kernels was 25.8, 30.6 and 13.3% respectively. The low values of HB2 implied that the concerning traits were easy to be effected by environments, especially for P content. Correlation analysis results of N, P, K contents in kernels on average of F2?4 / F2?5 populations showed that a notable positive relationship existed between N and P with r=0.229 and r=0.132 (r0.05=0.138, r0.01=0.181) under N- and N+ condition respectively, while no significant correlation had been found between N and K either under N- or N+ condition. It inferred that the lack of nitrogen had more affect on P than on K content in the kernels.
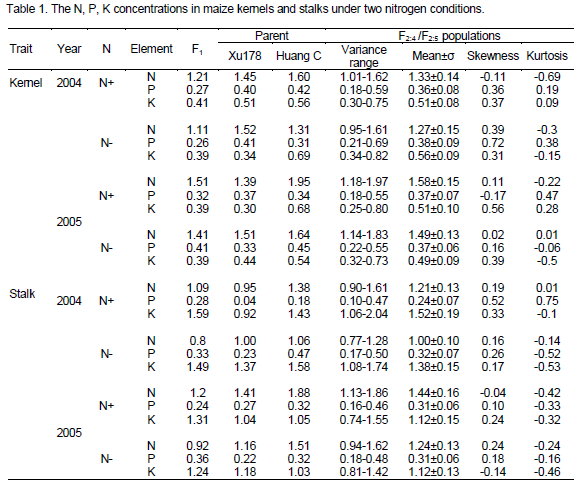
The N, P, K contents in maize stalks under two nitrogen conditions
The N and K contents in the stalk of F2?3 family lines were highly significant increased under N+ condition compared with N- condition (t= 14.73 and 3.47; t0.05=1.96, t0.01= 2.58), while P content obviously decreased (t=-8.98, Table 1). Also, the N content in the stalk of F2?4 family lines was highly significant increased (t=12.73), P content decreased with the same trend as in F2?3 family lines even though the difference was not significant statistically (t=-0.67), while no significant differences were found in the content of K (t=-0.01). The results showed that soil N level not only affect the maize absorption to N, but also affect the transportation of other elements such as P and K from stalk to grain in some degree. The heritability in broad sense of N, P and K contents in stalks was also low with 20.7, -9.8 and 15.4% respectively.
The correlation analysis results of N, P, K contents in stalks averagely of F2?4 / F2?5 populations showed that significant relationship existed between N and P under N+ condition (r=0.14), and between N and K under N- condition (r=-0.14) respectively. The results showed that nitrogen supply level in soil also affect the P and /or K contents in stalks in maize.
QTL detected for N, P, K contents in maize kernels
Out of the 235 SSR markers with polymorphism between both parents, 199 SSR markers were used to construct the genetic linkage map for the F2 population by means of Mapmaker 3.0. The genetic linkage map included 10 linkage groups, spanning a total of 2100.9 cm with an average interval of 10.82 cm (Figure 1).
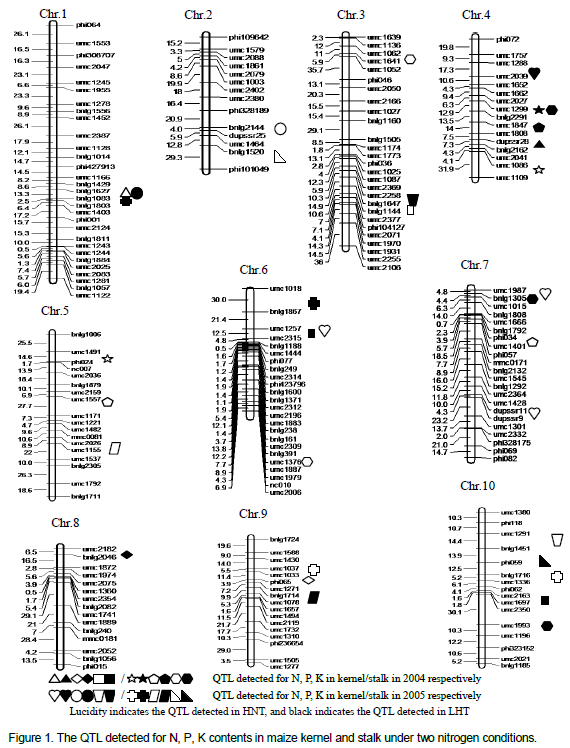
In total, fifteen QTL for N, P, K contents in maize kernels were detected using the composite interval mapping method under two N levels in 2004 and 2005. Of which, seven QTL were detected under N- condition, while eight were identified under N+ condition, the QTL located on all chromosomes except for Chro.5 (Table 2 and Figure 1). Among the 6 QTL for N content in maize kernels, 4 and 2 QTL were detected under N+ and N- treatments, respectively, and of which, one and one QTL were detected in 2004 and three as well as one QTL in 2005 under N+ and N- conditions respectively, located on chromosome 1, 4, 6 and 7. Single QTL could explain the phenotypic variation from 7.80 to 14.91%, the QTL qNC1b, qNC4c and qNC6b appeared increasing action from the loci of parent Xu178 and the other QTL from the loci of parent Huang C.
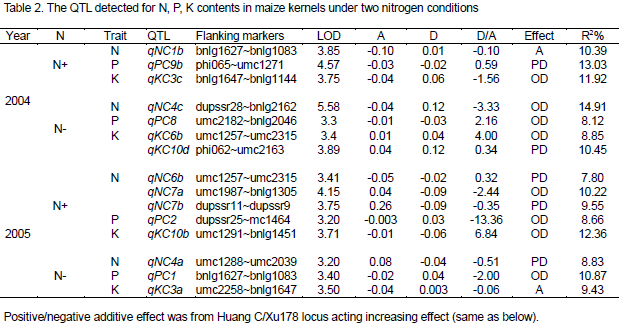
Out of the four QTL for P content in maize kernels, only one QTL was detected under two nitrogen conditions in two years respectively, with a contribution to phenotypic variance of 8.12 to 13.03%, all appeared increasing action from the loci of parent Xu178. Obviously, view from the point of nutrient compositions of maize grain, the parent Xu178 played main roles in P absorption and accumulation.
In the total of five QTL for K content in maize kernels, two and three QTL were detected under N+ and N- treatments, respectively, and of which, one and two were detected in 2004 and 2005 under two nitrogen conditions respectively, with a contribution to phenotypic variation from 8.85 to 12.36%. The QTL, qK10b, which detected under N+ condition in 2005, has a 12.36% contribution to K content in maize kernels. In these QTL detected for K content, the QTL qKC3a, qKC3 c and qKC10b was derived from the loci of parent Xu178, while qKC6b and qKC10d from Huang C performed increasing action to K content in maize kernels. Out of the fifteen QTL detected for N, P, K content under two nitrogen condition in two years (Table 2), two QTL appeared additive, five QTL exhibited partial dominance and eight QTL expressed over dominance.
QTL detected for N, P, K contents in maize stalks under two nitrogen conditions
There were nineteen QTL detected for stalk N, P, K contents under two N levels in 2004 and 2005. Of which, ten QTL detected under N+ condition, while nine under N- condition, located on all chromosomes except chromosome 8 (Table 3 and Figure 1). Among the seven QTL for N content in maize stalks, four and three QTL were detected under N+ and N- treatments, respectively, of which, two and one was detected in 2004 as well as each two in 2005 under N+ and N- conditions, lied on chromosome 1, 4, 5, 6, 9 and 10. Single QTL could explain the variance of phenotype ranged from 7.30 to 31.09%, and the QTL qNC4b has a 31.09% contribution to phenotypic variation of N content in stalks under N+ condition in 2004.
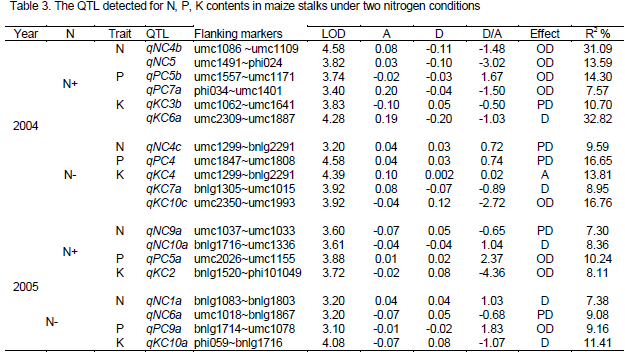
Out of the five QTL for P content in stalks, three and two QTL were detected under N+ and N- treatments, located on chromosome 4, 5, 7 and 9, with a contribution for phenotypic variation ranged from 8.12 to 13.03%. The QTL, qPC4 was an important QTL, with 16.65% contribution to the P content in stalks under N- condition in 2004.
In the total of seven QTL for K content in stalks, three and four QTL were detected under N+ and N- treatments respectively, and of which, two and three QTL were detected in 2004, while one and one was identified in 2005 under N+ and N- conditions, occupied on chromosome 2, 3, 4, 6, 7 and 10, with a contribution rate ranged from 8.85 to 32.82%. The qKC6a was a main QTL controlling the K content in stalks explaining the variance of phenotype by 32.82% under nitrogen condition in 2004.
Of the nineteen QTL detected for N, P, K content in maize stalks, one QTL appeared additive, five QTL expressed partial dominance, five and eight QTL exhibited dominance and over dominance. Obviously, over dominance was the main action of the loci controlling the N, P, K contents in maize stalks, followed by the QTL partial dominant and dominant effect. All from above, nine (about 1/2) were derived from parent Xu178 with only 38.55% increasing the action of total phenotypic variation (Table 3), while ten QTL derived from Huang C (about the rest 1/2) with 61.45% increasing the action of total phenotypic variation. The results demonstrated that the loci from the parent Huang C played important roles in N, P, K absorption while the loci from Xu178 played important roles in N, P, K transportation from stalk to grain.
Effect of low N stress to N, P, K nutrition balance
Although N, P and K are three important required mineral elements for crops, and play important roles on nutrient metabolism, growth and development. However, plant requires a certain ratio for different nutrient elements during its developing period normally. Lack of any nutrient element may induce plant disease symptom and affect the normal metabolism based on the equivalent important and irreplaceable principle (Liu, 2006). Besides, lack of any one nutrient element may break the balance between nutrient elements and lead to poor effect to plant in nutrient absorption and utilization. In fact, the N content in the soil in many areas of world is very absent, so low N stress not only reduces the concentration of N in grain and stalk, and affect grain yield, it also leads to increase or decrease the contents of P, K, that is, low N stress also affects the balance absorption and distribution of N, P, K in the stalks and kernels. But the variance ranges of nutrient contents tend to be narrower under N- condition based on the variance ranges of the three elements especially in kernels for F2?5 population and in the stalks for the F2?4 population (Table 1). Liu et al. (2006) have suggested that the average N: P: K ratio in the plant dry matter is about 6.0: 1: 5.4 for maize, in this study, the average ratio of N: P: K content in kernels and stalks of populations is about 5.1:1:1.9 and 3.6:1:4.0 under N- condition, as well as 4.0:1:1.5 and 4.8:1:4.8 under N+ condition, respectively. Observably, more N and K contents in stalks under N+ than N- condition, while inversion, more N and K contents in kernels under N- than N+ condition. The result showed that more N and K element are transferred from stalk to kernel in low nitrogen stress. Huang et al. (2004) have reported that the corn grain yields of combined application of N P K were significantly higher compared to P K, N K, and N P treatments, respectively. The grain yield in maize production which applied N, P and K could increase by 15.9, 6.9 and 12.1% for high-oil corn, as well as by 20.3, 8.6 and 12.7% for high-starch corn, respectively. Obviously, a proper ratio of N, P, K was essential for maize plant to get a higher yield, land the lack of N nutrient would reduce grain yield and affect the quality (Oikeh et al., 1998).
The genetic basis of nitrogen usage
People consider that conventional fertilizer recommendations result in higher than necessary costs to farmers and increased environment pollution (Zhang et al., 1995; Wu, 1997; Liu et al., 2006). Cai et al.?2002?thought that ammonia loss was an important pathway of N loss from fertilizer applied to maize (11 to 48%) in North Chinese Plain. So, the farmers must optimize the use of nitrogen (N) fertilizer in order to preserve their net income and to decrease pollution. To reach the objectives, specific farming techniques and varieties with a better NUE should be used synthetically for raising the economic in maize production (Bertin and Gallias, 2001). And as a whole, genetic variability was expressed differently in N+ and N- such as QTL for N- uptake were mainly detected in N+, whereas QTL for nitrogen utilization efficiency was mainly detected in N- (Bertin and Gallias, 2001; Gallais and Hirel, 2004), this suggests that the limiting steps in N- assimilation may be different when plants are grown under high or low levels of nitrogen fertilization. Based on the principle of nutrient elements equally important in plant nutrition metabolism, a proper ratio of N, P, K supply will maintain crops growing normally and lack of any one, such as under low N-stress, will affect the other nutrients uptake and transportation for the balance broken (Huang et al., 2004).
Gene expression and interaction with environment
Gene expression relies on given environments, the interaction between QTL and environment is one of the most important factors effecting quantitative traits, and so the QTL mapping results were different with conditions (Veldboom and Lee, 1996; Lübberstedt et al., 1997; Agrama et al., 1999; Bertin and Gallias, 2001; Tang et al., 2005b). Asíns (2002) reported that the variance of some quantitative traits was induced by the action of many genes together with environment. The QTL detected in this research for N, P, K contents were all only appeared in single condition, and showed variances distribution and genetic effect, which inferred that the inheritance of quality traits in maize was influenced by QTL and environments, similar results obtained in other researches on other traits in maize (Zhuang et al., 1997; Lan et al., 2005). So, digging QTL effecting in different environments will be more significance for associate breeding by molecule marker in maize.
The authors have not declared any conflict of interest.
This work was supported by National High Technology Research and Development Program of China (2012AA10A305) and Science and Technology Support Program of China (2011BAD35B01).
REFERENCES
Agrama HAS, Zakaria AG, Said FB, Tuinstra M (1999). Identification of quantitative trait loci for nitrogen use efficiency in maize. Mol. Breed. 5:187-195.
Crossref |
|
|
Aildson PD, Stephen CM, David SJ, Jorge de CK (2005). Grain quality of Brazilian maize genotypes as influenced by nitrogen level. Crop Sci. 45:1958–1964.
Crossref |
|
|
Asíns MJ (2002). Present and future of quantitative trait locus analysis in plant breeding. Plant Breed. 121(4):281-291.
Crossref |
|
|
|
Bao SD (2000). Analysis of soil agricultural chemical. Beijing, China Agricultural Press. |
|
|
|
Bertin P, Gallais A (2001). Genetic variation for nitrogen use efficiency in a set of recombinant inbred lines. II. QTL detection and coincidences. Maydica 46:53-68. |
|
|
Hirel B, Bertin P, Quilleré I, Bourdoncle W, Attagant C, Dellay C, Gouy A, Cadiou S, Retailliau C, Falque M, Gallais A (2001). Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 125(3):1258-1270.
Crossref |
|
|
Cai GX, Chen DL, Ding H, Pacholski A, Fan XH, Zhu ZL (2002). Nitrogen losses from fertilizer applied to maize,wheat and rice in the north China plain. Nutr. Cycl. Agroecosyst. 63:187-195.
Crossref |
|
|
|
Chen FJ, Mi GH, Zhang FS, Wang Y, Liu XS, Chun L (2003). Nitrogen use efficiency in some of main maize hybrids grown in north China. J. Maize Sci. 11(2):78-82. |
|
|
|
Churchill GA, Doerge RW (1994). Empirical threshold values for quantitative trait mapping. Genetics 138(3):963-971. |
|
|
|
Doerge RW, Churchill GA (1996). Permutation tests for multiple loci affecting a quantitative character. Genetics 142(1):285-294. |
|
|
Gallais A, Hirel B (2004). An approach to the genetics of nitrogen use efficiency in maize. J. Exper. Bot. 55(396):295-306.
Crossref |
|
|
|
Huang SW, Sun GF, Jin JY, Zuo YB, He P (2004). Plant Nutrition and Fertilizer. Science 10(3):225-230. |
|
|
Knapp SJ, Stroup WW, Ross WM (1985). Exact confidence intervals for heritability on a progeny mean basis. Crop Sci. 25:192-194.
Crossref |
|
|
|
Lan JH, Li XH, Gao SR, Zhang BS, Zhang SH (2005). QTL analysis of yield components in maize under different environments. Acta Agronom. Sin. 31(10):1253-1259. |
|
|
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987). Map Maker: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1:174-181.
Crossref |
|
|
|
Liu CS (2006). Soil and Fertilizer, China Agricultural University Press, Beijing pp.146-148. |
|
|
Liu MQ, Yu ZR, Yun H, Konijn NT (2006). Fertilizer requirements for wheat and maize in China: the QUEFTS approach. Nutr. Cycl. Agroecosyst. 74:245-258.
Crossref |
|
|
Liu ZH, Xie HL, Tian GW, Chen SJ, Wang CL, Hu YM, Tang JH (2008). QTL mapping of nutrient components in maize kernels under low nitrogen conditions. Plant Breed. 127:279-285.
Crossref |
|
|
Lübberstedt T, Melchinger A E, SchÇ’n CC, Utz HF, Klein D (1997). QTL mapping in testcrosses of European flint lines of maize: I. Comparison of different testers for forage yield traits. Crop Sci. 37:921-931.
Crossref |
|
|
|
Machado AT, Magalhaes JR, Magnavaca R, Silva MR (1992). Activity of enzymes involved in the nitrogen metabolism in different maize genotypes. Rev. Bras. Fisiol. Vegetal. 4(1):45-47. |
|
|
|
Martín U, Steven JCB, Fred EB (2008). Physiological N response of field-grown maize hybrids (Zea mays L.) with divergent yield potential and grain protein concentration. Plant Soil 316:151-160. |
|
|
Monneveux P, Zaidi PH, Sanchez C (2005). Population density and low nitrogen affects yield-associated traits in tropical maize. Crop Sci. 45:535-545.
Crossref |
|
|
Oikeh SO, Kling JG, Okoruwa AE (1998). Nitrogen fertilizer management effects on maize grain quality in the west African Moist Savanna. Crop Sci. 38(4):1056-1061.
Crossref |
|
|
Pan WL, Camberato JJ, Moll RH, Kamprath EJ, Jackson WA (1995). Altering source-sink relationships in prolific maize hybrids: Consequences for nitrogen uptake and remobilization. Crop Sci. 35:836-845.
Crossref |
|
|
Presterl T, Seitz G, Landbeck M, Thiemt EM, Schmidt W, Geiger HH (2003). Improving nitrogen-use efficiency in European maize: estimation of quantitative genetic parameters. Crop Sci. 43:1259-1265.
Crossref |
|
|
|
Raja V (2001). Effect of nitrogen and plant population on yield and quality of super sweet corn. Indian J. Agron. 46(2):246-249. |
|
|
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984). Ribosomal DNA spacer length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population, and population dynamics. Proc. Natl. Acad. Sci. USA. 81(17):8014-8018.
Crossref |
|
|
Stuber CW, Edwards MD, Wendel JF (1987). Molecular marker-facilitated investigation of quantitative trait loci in maize II. Factors influencing yield and its component traits. Crop Sci. 27:639-648.
Crossref |
|
|
|
Tang H, Yan JB, Huang YQ, Zheng YL, Li JS (2005a). QTL mapping of five agronomic traits in maize. Acta Genet. Sin. 32(2):203-209. |
|
|
|
Tang JH, Xie HL, Huang SM, Hu YM, Liu ZH, Ji HQ, Kou ZA (2005b). The Changes of the Content for Chlorophyll and Photosynthetic Productivity in Maize Inbred Lines under the Low-nitrogen Stress. Acta Agric. Boraeali-Sin. 20(5):10-12. |
|
|
|
Teng Y, Zhang ZX, Wei YX, Wang ZB, Wang MX (2005). Effect of soybean yield and soil water in semiarid district of northeast under the different ratios of N, P and K. J. Northeast Agric. Univ. 36(3):273-279. |
|
|
Veldboom LR, Lee M (1996). Genetic mapping of quantitative trait loci in maize in stress and nonstress environments: Grain yield and yield components. Crop Sci. 36:1310-1319.
Crossref |
|
|
|
Wang S, Basten CJ, Zeng ZB (2004). Windows QTL Cartographer 2.0. Department of Statistics, North Carolina State University, Raleigh, NC. |
|
|
|
Wu ZJ (1997). Problems in production and utilization of China's chemical fertilizer and their solutions. Sci. Technol. Rev. 9:37-39. |
|
|
|
Zeng ZB (1994). Precision mapping of quantitative trait loci. Genetics 136(4):1457-1468. |
|
|
|
Zhang FS, Mi GH, Liu JA (1997). Advances in the genetic improvement of nitrogen efficiency in maize. J. Agric. Biotechnol. 5(2):112-117. |
|
|
|
Zhang WL, Tian ZX, Zhang N, Li XQ (1995). Investigation of nitrate pollution in ground water due to nitrogen fertilization in agriculture in North China. Plant Nutr. Fert. Sci. 1(2):80-87. |
|
|
Zhu ZL, Chen DL (2002). Nitrogen fertilizer use in China- contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 63:117-127.
Crossref |
|
|
Zhuang JY, Lin HX, Lu J (1997). Analysis of QTL x environment interaction for yield components and plant height in rice. Theor. Appl. Genet. 95:799-808.
Crossref |



