ABSTRACT
In the tropics, high temperatures and nutrient inaccessibility are often the most limiting factors affecting plant growth and final crop yield. However, it remain incomprehensible the actual impact of simultaneous interaction of prior heat stress and nutrient addition on growth and yield of cowpea plant, knowing that cowpea plant is an important grain legumes for over 200 million people in dry savanna of tropical Africa. In this study, the effect of interaction between prior heat stress and nutrient addition on growth and yield of cowpea plant was carried out in a randomized pot experiment at Obafemi Awolowo University, Ile-Ife, Nigeria. Cowpea seedlings were exposed to prior heat stress of 40°C a week after germination and different level of nutrient addition. The result obtained from the study showed that there was no significant (p<0.05) difference in the interactive effect of nutrient addition and prior heat stress on the shoot height of cowpea in most of the treatments compared to control, except in NNH3 (200 ml of the nutrient solution on daily basis and 3 h of prior heat stress) and N5H6 (200 ml of nutrient in every five days and six hours of prior heat stress). The shoot height of N5H6 was significantly higher (41.17) than NNH3 (20.26 cm). Cowpea plant subjected to only water with no prior heat stress had the highest leaf dry weight (2.44 g), while the cowpea plant under the interactive effect of nutrient supplied at every five days and six hours of prior heat stress had the highest shoot dry weight (2.63 g). The feedback of cowpea plant to interactive effect of prior heat stress and nutrient addition indicated that Longer period of prior heat stress had better advantages on growth parameter cowpea plant. However, neither prior heat stress nor nutrients addition had significant benefit on the leaf yield of cowpea, the interaction of the two significantly benefit shoot biomass and pod number of Cowpea plant.
Key words: Cowpea, growth, nutrient. heat, nodule, stress, treatment, yield.
The frequency of extreme stress event on plant growth, development and yield differ among varieties of plant species, depending on the prevailing optimal environments as well as the susceptibility capacity of individual species to a particular stress type. Commonly, plants are considered to be under stress when they experience a relatively severe scarcity of an essential element or an excess of a potentially toxic or damaging substance (Okunlola and Adelusi, 2012). Nevertheless, the ability of individual plant species to tolerate multiple stresses through morphological adjustments is a key feature that regulates species survival and colonization, and hence the ecological breadth of the species (Bazzaz, 1996; Sultan et al., 1998). Temperature is one of the major prevailing factors affecting the life of all living organisms. At the cellular level, heat stress beyond a threshold level plays a critical role in affecting the constancy of various proteins, fluidity of membrane lipids and metabolic imbalance (McClung and Davis, 2010; Wahid, 2007). Heat as a complex function of intensity, duration and rate of increase in temperature can cause declines in photosynthetic efficiency, leaf senescence, shoot and root growth inhibition, impairment of pollen and anther development, and subsequently reduction in yields (Karim et al., 1999; Sato et al., 2006; Wahid, 2007; Zhang et al., 2005). Heat stress due to high temperatures is a crucial threat to crop production worldwide (Hall, 2001) as it causes reduction in yield and dry matter production in many crops (Giaveno and Ferrero, 2003).
Around the world, nutrient addition is considered one of the major factor, to linearly increase crop production. However, sub-optimal availability of essential nutrients or toxicity of nutrient or non-nutrient minerals may pose primary restraint to plant growth and development over the majority of the earth’s land surface (Okunlola et al., 2015). For instance, nitrogen being the most important macronutrient for plant due to it essential component in plant cell compounds such as chlorophyll and proteins (Srivastava and Singh, 1999), which are closely associated with leaf colour, crop growth status and yield (Fageria et al., 2011), constitutes only 2 to 4% of plant dry matter. Thus, it deficiency or supererogatory can affect leaf growth, leaf area, leaf duration, the photosynthetic rate per leaf area, and the size of the vegetative storage organs (Abdelhalim et al., 2016), thereby affecting crop yields negatively.
Despite the vast array of research on the effect of stress on crop growth, development and yields, the interactive effect of different type of stress on food crop are still rare. Particularly, the interactive effect of prior heat stress and nutrient addition on growth, development and yield of Cowpea plant (Vigna unguiculata (L.) Walp) has not been well researched. Thus, it is important to understand the interactive effect of prior heat and nutrient stress on the overall productivity of cowpea, being an economically viable food crops. Cowpea plant (V. unguiculata (L.) Walp.), is an annual bushy herb plant with erect, creeping or climbing stems depending on the varieties, (Ferreira, 2004; Shao et al., 2008). V. unguiculata is an important economic seed legumes in many developing regions due to its high protein content, resistance to drought, adaptability to different types of soil and intercropping systems (Shao et al., 2008). It is also desirable in that it has little dependence on chemical fertilizer for optimum yield if successfully nodulated with appropriate Rhizobium (Abayomi et al., 2008). Several studies such as Sunita and Uma (1993), Sharma and Gill (1994), Shinozaki and Yamaguchi-Shinozaki (2000) have reported the effect of different single stress factors on growth and yield of cowpea. However, the response of cowpea growth, development and yield to interactive effect of multiple stress factors has not been adequately established, especially in the savannah ecological zone which is the principal area of cultivation.
Therefore, in order to close this gap in knowledge, the present study evaluate the magnitude of effect due to simultaneous interaction of prior heat and nutrient stress on growth, development and yield of cowpea. We theorized that (i) the interaction of moderate or no prior heat stress and nutrient addition will significantly improve the overall productivities of cowpea plant, this was with the aim that optimal availability of essential nutrients will negate the effect of prior heat stress, (ii) at higher prior heat stress, the overall productivities of cowpea plant will significantly decline.
Experimental design
A randomized pot experiment was conducted at the premises of Department of Botany, Obafemi Awolowo University, Ile-Ife. Topsoil was collected from a re-growth forest behind the Biological garden of Obafemi Awolowo University, Ile Ife, Nigeria. The soil samples were thoroughly mixed to obtain homogeneity and later sieved to remove the particles other than soil. Three wooden pots of approximately 30 cm x 30 cm were filled with the soil (Okunlola and Adelusi, 2013) and seeds of V. unguiculata were sown at a depth of about 1cm. The three wooden pots were divided into three regimes of (i) no heat stress (HO), (ii) 3 h prior heat stress (H3) and (iii) 6 h prior heat stress (H6). The seedlings were allowed to germinate in the open environment for a period of one week and, then subjected to prior heat stress using Gallenkamp illuminated cooled incubator at 40°C (Okunlola and Adelusi, 2013). The first regime was incubated for three hours while the second regime was incubated for six hours. At the end of the incubation period, all the three wooden pots were transferred into the screen house. Thirty-six plastic pots were filled with the topsoil, with holes at the base of each plastic pot to ensure drainage of excess water. In order to further unravel the possible interaction between prior heat and nutrient addition, the experiment was further categorized into twelve (12) treatments based on the combined factors of the duration of nutrient supplied; no heat stress and prior heat stress (Table 1).The nutrient solution was prepared in accordance with the modified long Ashton formula (Hewitt, 1952).
Determination of growth and yield parameters
During the growing period, measurement of some growth parameter such as leaf area, {leaf length and leaf breath}, leaf number and shoot height were carried out up to the period of harvesting. Leaf area was calculated using the formula of Osei-Yeboah et al. (1983); A= LxWx2.325. In order to determine the yield response of V. unguiculata to treatments, each of the plant was carefully harvested. The dry weight of leaves, stems, roots and pods were determined after oven dried at 80°C. The pod number and root nodules were also estimated.
Statistical analysis
The result obtained from the study was subjected to both inferential and descriptive statistical analysis The effect of different treatments on each growth parameter were tested using Analysis of variance (ANOVA), least significant different (LSD) was used to evaluate the difference between separated means under each treatment. Single linkage cluster analysis was also performed on the selected yield parameter in other to show the likely similarity among the treatments. All statistical analysis were performed using SAS (SAS system for window 8) and PAST 3.01 software.
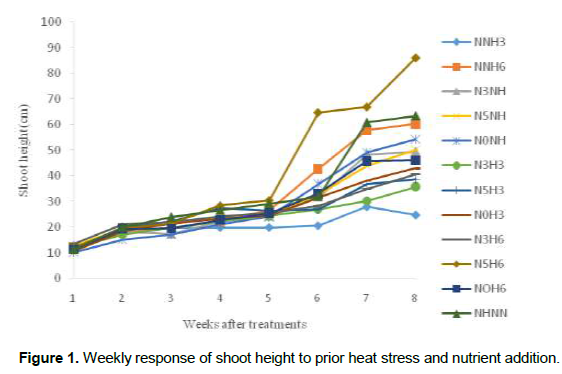
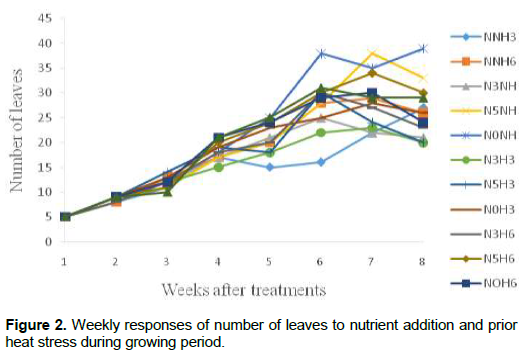
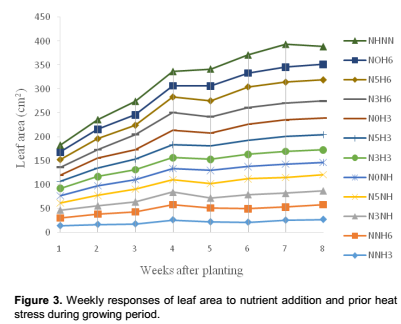
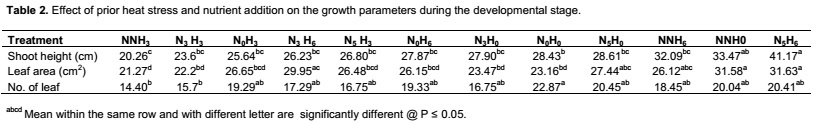
Growth and developmental characteristics of Vigna unguiculata under interactive effect of prior heat stress and nutrient addition Evaluating the interactive effect of nutrient addition and prior heat stress on shoot height, leaf area and number of leaves of V. unguiculata during growing period, our result revealed that there was a progressive increase in the number of leaves, leaf area and shoot height from the first week (week 1) of the nutrient treatment to week 8 (Figures 1, 2 and 3). In addition, the result of the ANOVA revealed that there was no significant difference in the interactive effect of nutrition addition and prior heat stress on the shoot height of V. unguiculata in most of the treatments compared to control, except in NNH3 and N5H6 which are significantly different from each other and also from the control (Table 2). The shoot height recorded when 200 ml of the nutrient solution was applied on daily basis to 3 h prior heat stress (NNH3) V. unguiculata plant was significant difference (P<0.05) from V. unguiculata subjected to 200 ml of nutrient in every five days and six hours of prior heat stress. Particularly, the shoot height of N5H6 was significantly higher (41.17) than NNH3 (20.26). Similar to the observed trend in shoot height, the leaf area of V. unguiculata when subjected to daily nutrient supply (everyday) with no prior heat stress (NNH0) and when subjected to 200 ml of nutrient in every five days and six hours of prior heat stress (N5H6) treatments was significantly higher (P<0.05) than NNH3. The average leaf area recorded during the study period range from 21.27 in NNH3 treatment to 31.63 in N5H6 treatment. Furthermore, there was no significant different in the number of leaf recorded among the treatments. However, there was a decreased in the total number of leaf at NNH3 (14.40) and N3H3 (15.7) treatments (Table 2).
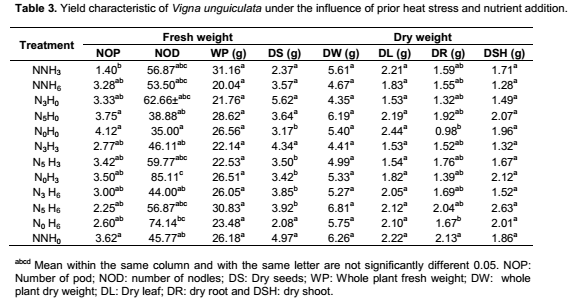
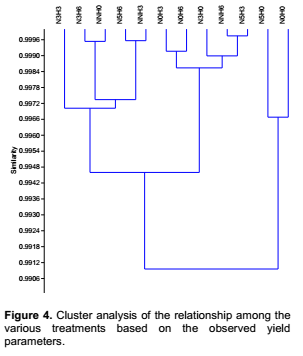
Yield characteristic of Vigna unguiculata under the interactive influence of prior heat stress and nutrient additionAt harvest, there was no significant interaction of prior heat stress and nutrient addition majority on the yield parameter evaluated (Table 3), except in few treatments. The total pod number recorded among treatments were not significantly different from each other. Nevertheless, the pod number recorded when 200 ml of nutrient solution interact on daily basis with 3 hours of prior heat stress (NNH3) (1.40) was significantly (p<0.05) different from the total pod number recorded at N5H0, N0H0 and NNH0 (3.75, 4.75 and 3.62) treatments respectively (Table 3). V. unguiculata subjected to three hours of prior heat stress with no nutrient supply (N0H3) had significantly (p<0.05) higher number of nodules than the number ofnodules recorded among other treatments. Although there was no significant (p<0.05) different in the total leaf biomass (leaf dry weight) and shoot biomass (shoot dry weight) among the treatments, however, V. unguiculata subjected to only water with no prior heat stress had the highest leaf dry weight (2.44), while the V. unguiculata under the interactive effect of 200 ml of nutrient supplied in every five days to six hours of prior heat stress plant had the highest shoot dry weight (2.63). Furthermore, V. unguiculata under the interactive effect of 200 ml of nutrient solution supplied every three day with no heat stress (N3H0) and 200 ml of nutrient solution supplied every three day with three hours of prior heat stress recorded the highest seed biomass (Table 3). In addition, the result of this study further showed that cowpea plant under NNH6 treatment had higher pod number (3.28) compared to cowpea plant under NNH3 (1.40) treatment. In contrary, N5H3 had higher number of nodules (3.42) compared to N5H6 (2.25). The result of the cluster analysis further revealed that V. unguiculata under the interactive influence 200ml of nutrient solution supplied in every five days with no prior heat stress N5H0 and those supplied with only water and also with no prior heat stress (N0H0) are more related to each other, but distantly related to the other treatments and cluster together with similarity index of above 90% (Figure 4).
The extent to which stress affect plant is a complex process. While high light intensity may induce severe photo-oxidative damage to chloroplasts, and consequently cause decrease in the yield capacity of plants, the mineral nutritional status of plants may significantly affects their ability to acclimatize to adverse environmental conditions (Okunlola et al., 2015; Okunlola and Adelusi, 2014). In the present study, the combine effect of different hours of prior heat stress and different nutrient addition level have dissimilar effect on the overall growth and yield characteristic of cowpea plant. While some treatment do not significantly affect the growth and yield parameters of cowpea, other posed significant (p<0.05) effects. For instance, when 200 ml of the nutrient solution supplied on daily basis interacted with 3 h of prior heat stress, the shoot height and leaf area was significantly reduced. However, when 200 ml of nutrient supplied every five days interacted with six hours of prior heat stress, shoot height, leaf area and number of leaves significantly increased. Since the prior heat stress used in the present study is below the temperature at which plant tissues is expected to damaged (46.1°C), thus, the present observation suggested that longer period of prior heat stress have better advantages on cowpea plant, even when nutrient was not supplied or inadequately supplied. According to Thompson et al. (1988), medium irradiance and high nutrient levels will result into optimal leaf expansion, Chlorophyll content and Photosynthesis. Furthermore, the observed higher growth parameter when nutrient was supplied on daily basis with no prior heat stress agreed with our hypothesis that the interaction of moderate or no prior heat stress and nutrient addition will significantly improve the overall productivities of cowpea plant. The present findings suggested the ability of cowpea plant to ameliorate the equivocal effect of prior heat stress when interacted with nutrient addition. This agreed with the finding of Okunlola and Adelusi (2014) that plants grown at high nutrient availability generally produce larger leaves and greater allocation to phosynthetic protein than those at low nutrient availability.
According to Jurik (1991), variation in temperature will greatly affects plant growth and development, therefore, the low leaf area observed when nutrient solution supplied on daily basis interacted with 3 h of prior heat stress and when nutrient solution supplied every three days interacted with 3 h of prior heat stress maybe attributed to the combine effect of nutrient and prior heat stress which may impede the production of leaf. This agreed with Azcon-Bieto and Osmond (1983) that nutrient stress affects the development of mesophyll cell which make up the bulk of the internal leaf tissue and also affect leaf development. Furthermore, the high pod number observed when nutrient was supplied to cowpea plant without prior heat stress compared to cowpea plant under prior heat stress may be attributed to absence of heat stress in those treatments. According to Ghosh et al. (2000) and Sato (2006) subjecting plant to either moderate or intense heat stress will resulted into severe yield loss and subsequently extended into total crop failure. Reproductive development of many crop species is damaged by heat such that they produce no flowers or if they produce flowers they may set no fruit or seeds (Hall, 1993).
Crop sensitivity to heat stress hinge on the length of anthesis and also on the duration of heat stress (Hatfield and Prueger, 2015). The present findings revealed that longer duration of prior heat stress when interacted with daily addition of nutrient solution resulted in higher pod number in cowpea plants. However, interaction of longer prior heat stress (six hours) with nutrient addition at every five days interval resulted into decreased in number of nodules compared to three hours of prior heat stress. This suggested that duration of prior heat stress may impact the fertility of cowpea flower and subsequently impacting the pod yield of cowpea plant. Generally, both prior heat and nutrient addition significantly increased number of nodules in cowpea plant compared to when neither prior heat stress nor nutrient was applied. This finding corroborated the work of Simoes-Araujo et al. (2008) that in the tropical area, cowpea nodules are very resistant to heat and high temperature stress and, thus enhanced the number of nodules produced. Abdel-Wahaab et al. (1994) and Norman et al. (1995) reported that nutrients addition, particularly phosphorus will stimulate root and plant growth, initiate nodules formation and also influences efficiency of rhizobium-legumes symbiosis, consequently optimizing the biological nitrogen fixation systems of legumes.
The significant of parameters like shoot, plant height, leave biomass and total plant biomass as measure of yield had been suggested in several study (Hatfield and Prueger, 2015), therefore they may be used as a measure of yield response of cowpea to prior heat stress and nutrient addition. Although there was no significant (p<0.05) different in the total leaf biomass (leaf dry weight) among the treatments, however, the leaf biomass of V. unguiculata subjected to only water addition with no prior heat stress and nutrient addition had the highest leaf dry weight. This suggested that neither prior heat stress nor nutrients addition had significant benefit on the leaf yield of cowpea plant. furthermore, the shoot biomass of V. unguiculata under the interactive effect of nutrient supplied at every five days and six hours of prior heat stress was highest compared to other treatment. In general, among the different yield parameter studied leaf biomass had the highest dry weight in all the treatment. This may be attributed to food production capacity of the leaf, since the leaf is the site for food manufacture through photosynthesis.
The interactive effect of prior heat stress and nutrient addition on cowpea plant was quite universal. Longer period of prior heat stress have better advantages on growth parameter of cowpea plant, even when nutrient was not or inadequately supplied compared to shorter or no prior heat stress. It can further be concluded that while neither prior heat stress nor nutrients addition had significant benefit on the leaf yield of cowpea plant, the interaction of the two significantly benefit shoot biomass and pod number of cowpea plant. However, interaction of longer prior heat stress (six hours) with inadequate nutrient addition decreased number of nodules compared to shorter hours of prior heat stress. To obtain comprehensive overview of growth and yield response of cowpea plant, we suggested further studies to elucidate the physiological basis for growth and yield responses in cowpea plant under the interactive effect of prior heat stress and nutrients addition. We further suggested that the potential adaption mechanism underline the response of cowpea plant to interactive effect of different stress factor should be determined.
The authors have not declared any conflict of interests.
REFERENCES
|
Abayomi YA, Ajibade TV, Samuel OF, Sa'adudeen BF (2008). Growth and yield response of cowpea (Vigna unguiculata (L).Walp) to Nitrogen fertilizer (NPK) Application in the southern Guinea Zone of Nigeria. Asian J. Plant Sci. 7(2):170-176.
Crossref
|
|
|
|
Abdelhalim E, Raziel AO, Roxana S, Gustavo AS, Jose LA (2016). Detecting interactive effects of N fertilization and heat stress on maize productivity by remote sensing techniques. Eur. J. Agron. 73:11–24.
Crossref
|
|
|
|
|
Abdel-Wahaab SM, Hassman ME, Elwarraky K, Safwat MSA (1994). Evaluation of N2-fixation of faba beans, chickpea and lentil as affected by phosphorus fertilization. Afr. Crop Sci. Conf. Proc. 1:71-73.
|
|
|
|
|
Azcon-Bieto J, Osmond CB (1983). Relationship between photosynthesis and respiration. The effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol. 71:617-630.
Crossref
|
|
|
|
|
Bazzaz FA (1996). Plants in changing environments: linking physiological, population and community ecology. Cambridge: Cambridge University Press.
|
|
|
|
|
Fageria NK, Baligar VC, Jones CA (2011). Growth and Mineral Nutrition of Field Crops. CRC Press, Boca Raton.
|
|
|
|
|
Ferreira FR (2004). Effect of different levels of water deficit on yield of groundnut, cowpea and maize. Thesis, college Agric. For., Maputo. P 334.
|
|
|
|
|
Giaveno C, Ferrero J (2003). Introduction of tropical maize genotypes to increase silage production in the central area of Santa Fe, Argentina. Crop Breed. Appl. Biotechnol. 3:89-94.
Crossref
|
|
|
|
|
Gosh SCA, Koh I, Kusutani A, Toyota M (2000). Effect of temperature at different growth stages on nonstructural carbohydrate, nitrate reductase activities and yield of potato. Environ. Control Biol. 38:198-206.
Crossref
|
|
|
|
|
Hall AE (1993). Physiology and breeding for heat tolerance in cowpea, and comparison with other crops, In: CG. Kuo (ed.) Adaptation of Food pp. 271-284.
|
|
|
|
|
Hall AE (2001). Heat Stress and its Impact.
View
|
|
|
|
|
Hatfield JL, Prueger JH (2015). Temperature Extreem: Effect on plant Growth and development. Weather Clim. Extremes 10:4-10.
Crossref
|
|
|
|
|
Hewitt EJ (1952). Sand and Water Culture Methods Used in The Study of Plant Nutrition. Commonwealth Agricultural Bureaux, Government Printer, HMSO.
|
|
|
|
|
Jurik TW (1991). Population distributions of plant size and light environment of giant ragweed (Ambrosia trifida L.) at three densities. Oecologia 87:539-550.
Crossref
|
|
|
|
|
Karim MA, Fracheboud Y, Stamp P (1999). Photosynthetic activity of developing leavea mays is less affected by heat stress than that of developed leaves. Physiol. Plant 105:685-693.
Crossref
|
|
|
|
|
McClung CR, Davis SJ (2010). Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Curr. Biol. 20:R1086-R1092.
Crossref
|
|
|
|
|
Norman MTJ, Perarson CJ, Seales PGE (1995). The Ecology of Tropical Food Crop. Cambridge University Press P. 65.
Crossref
|
|
|
|
|
Okunlola GO, Adelusi AA (2012) Effects of nutrient and light stress on some morphological parameters of tomato (lycopersicum esculentum mill). Ife J. Sci. 14(2):289-296.
|
|
|
|
|
Okunlola, GO, Adelusi AA (2013). Effect of Prior Heat Stress on the Early Growth of. Not. Sci. Biol. 5(4):508.
|
|
|
|
|
Okunlola GO, Adelusi AA (2014). Growth and Photosynthetic Pigment Accumulation in Lycopersicum esculentum in Response to Light and Nutrient Stress. Not. Sci. Biol. 6(2):250-255.
Crossref
|
|
|
|
|
Okunlola GO, Olatunji OA, Afolabi AM, Gbadegesin KK (2015). Effects of nitrogen nutritional stress on the morphological and yield parameters of tomato (Solanum lycopersicum L.). Sci. Cold Arid Regions 7(2):0137-0145.
|
|
|
|
|
Osei-Yeboah S, Lindsay JI, Gumb SFA (1983). Estimating leaf area of cowpea (Vigna ungiculata (L.) Walp) from linear measurement of terminal leaflets. Trop. Agr. 60(2):149-150.
|
|
|
|
|
Sato S (2006). The effects of moderately elevated temperature stress due to global warming on yield and the male reproductive development of tomato (Lycopersicu esculentum. mill.). Hortic. Res. 60:85-89.
|
|
|
|
|
Sato S, Kamiyama M, Iwata T, Makita N, Furukawa H, Ikeda H (2006). Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 97:731-738.
Crossref
|
|
|
|
|
Shao HB, Chud LY, Jaleelc CA, Zhaoe CX (2008). Water deficit stress induces anatomical changes. CR Biol. 331:215-225.
Crossref
|
|
|
|
|
Sharma PC, Gill KS (1994). Salinity induced effect on biomass, yield, yield attributing characters and ionic contents in genotypes of Indian mustard (Brassica juncea). Indian J. Agric. Sci. 64:785.
|
|
|
|
|
Shinozaki K, Yamaguchi-Shinozaki K (2000). Molecular responses to dehydration and low temperatures: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 3:217-223.
Crossref
|
|
|
|
|
Simoes-Araujo JL, Alves-Ferreira M, Rumjanek NJ, Margis-Pinheiro M (2008). VuNIP1 (NOD26-like) and VuHSP17.7 gene expression are regulated in response to heat Stress in cowpea nodule. Environ. Exp. Bot. 63:256-265.
Crossref
|
|
|
|
|
Srivastava HS, Singh RP (1999). Nitrogen Nutrition and Plant Growth. Sci. Publishers Inc., New Hampshire.
|
|
|
|
|
Sultan SE, Wilczek AM, Bell DL, Hand G (1998). Physiological response to complex environments in annual Polygonum species of contrasting ecological breadth. Oecologia 115:564-578.
Crossref
|
|
|
|
|
Sunita M, Uma M (1993). Environment and Agroforestry. Indian Farmers Digest. 25(3):29-30.
|
|
|
|
|
Thompson WA, Stocker GC, Kriedemann PE (1988). Growth and photosynthetic response to light and nutrients of Flindersia brayleyana F. Muell, a rainforest tree with broad tolerance to sun and shade. Aust. J. Plant Physiol. 15:299-315.
Crossref
|
|
|
|
|
Wahid A, Gelani S, Ashraf M, Foolad MR (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61:199-223.
Crossref
|
|
|
|
|
Zhang JH, Huang WD, Liu YP, Pan QH (2005). Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under cross-temperature stresses. J. Integr. Plant Biol. 47:959-970.
Crossref
|
|