ABSTRACT
Use of plant compounds in grain protection has shown great potential as an alternative to synthetic insecticides in Sub-Saharan Africa. This study investigated the efficacy of Eucalyptus grandis and Tagetes minuta ground leaf powders as grain protectants against Sitophilus zeamais in stored maize. Effect of leaf powders were evaluated on percent germination, percent weight loss of grain, insect infestation, grain colour and odour over 192 days (≈six months) duration. Leaf powders (2.5 and 5 g/kg), synthetic pesticide (Actellic Chirindamatura dust (0.5 g/kg)) and an untreated control were used as treatments. All plant powders significantly minimized grain damage and infestation 96 days post treatment (≈three months) and had no effect on percent germination of maize grains when compared to controls. However, variable responses dependent upon botanical plant cultivars and rate of application were observed from three to six months after application. Grain colour and odour were not affected by plant powders over six months of storage. E. grandis and T. minuta significantly reduce grain damage and insect infestation with no adverse effects on seed germination, colour and odour hence can be used as sustainable alternatives to synthetic insecticides in maize storage especially by smallholder farmers.
Key words: Sitophilus zeamais, efficacy, Eucalyptus grandis, Tagetes minuta, maize, organoleptic.
Maize is one of the major cereal grain produced by most small holder farmers in the Sub-Saharan African region and is critical in stimulating economic growth (Adetunji, 2007). The seasonal nature of its production in many African countries where one rainy season is experienced per year necessitates the requirement for good maize storage systems (Owusu et al., 2007). However, maize storage is constrained by a number of factors which include attack from pathogens and insect pests. Insect pests are the major threat, destroying approximately 20 to 50% of stored maize in most African countries (CABI, 2012; Dhliwayo and Pixley, 2003; Nukenine et al., 2002; Derera et al., 2001; Golop and Hodges, 1982).
In addition to destruction of grains by feeding and reproduction, insects cause an increase in grain temperature and moisture content. These lead to increased respiration and consequent loss in quality of the grain (Tefera et al., 2011; Caneppele et al., 2003). This also pre-disposes the grain to secondary attack by disease causing pathogens such as Aspergillus flavus Link leading to production of mycotoxins (Beti et al., 1995; Freer et al., 1990).
The maize weevil, Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae), is a serious pest of maize. In Zimbabwe, the postharvest losses due to S. zeamais have been recognized as an important constraint, with grain losses ranging from 20 to 90% being reported for stored untreated maize (Dhliwayo and Pixley, 2003). The grain damage caused affects both farmers and traders. S. zeamais larvae are internal feeders on the maize grains (CABI, 2012). Internal feeding affects seed viability thus negatively affecting seed germination where non-hybrid (retained seed) is used for new season planting.
Different technologies such as environmental manipulations to hinder growth, maturation and reproduction of storage pests have been effectively used (Moreno-Martinez et al., 2000, Oduor et al., 2000; Peng et al., 2000; Toscano et al., 1999; Thorpe, 1997; Maier et al., 1996). Such environmental manipulations have been attained by employing a number of control measures, including the use of pesticides, cultural and physical control measures (CABI, 2012; Pereira et al., 2009). Pesticides are effectively used against postharvest insect pests but are often associated with a number of drawbacks (Mulungu et al., 2010; Huang and Subramanyam, 2007; Benhalima et al., 2004).
Although S. zeamais can be effectively controlled by synthetic insecticides such as Shumba Super® 200G (Fenitrothion 1% Deltamethrin 0.13%) and Actellic Gold Chirindamatura® Dust (Pirimiphos-methyl 16 g/kg mass/mass and Thiamexotham 3.6 g/kg mass/mass) (Mashavakure, 2012; Mulungu et al., 2010) the majority of farmers in developing countries are resource poor and have neither the means nor the skill to obtain and handle pesticides appropriately (Kamanula et al., 2010). The increasing costs of application of the currently used synthetic pesticides, poor information and the often erratic supply of insecticides have emerged as the reasons for the farmers’ reluctance to use pesticides (Asawalam and Hassanali, 2006). The perception that pesticide residues in the food supply constitute a serious health risk and the development of insecticide resistance is a big concern in agricultural production. These concerns also raise a need for alternatives to grain protectants and eco-friendly insect pest control methods among which are the use of botanical pesticides (Asawalam and Arukwe, 2004; Bekele, 2002).
A number of botanical grain protectants in powdered form are used to reduce weevil damages in Zimbabwe. These include Lippia javanica L. (Gadzirayi et al., 2006), Lantana camara L. (Fusire, 2008) and T. minuta (Muzemu et al., 2013) leaves. Several studies have investigated the efficacy of Eucalyptus spp. leaves as grain protectants with many showing a high degree of effectiveness against major storage pests such as S. zeamais (Muzemu et al., 2013; Mulungu et al., 2007; Modgil and Samuels, 1998). Similarly, several studies have investigated the insecticidal properties of T. minuta against storage pests (Muzemu et al., 2013; Shahzadi et al., 2010; Weaver et al., 1994). However, very few of these studies have investigated the effect of these often strong smelling botanical plants on treated grain properties. One of the major concerns regarding use of insecticidal plants to control grain storage pests is the perceived fear that these products can adversely affect the taste, aroma and overall acceptability of treated grain (Ogendo et al., 2004). This study evaluated both the insecticidal and organoleptic properties of ground powders of E. grandis and T. minuta in stored maize grain.
Determination of efficacy of plants with pesticidal properties is one of the key steps in the bio-prospecting of new plant based compounds for grain protection. Although most recent advances in pesticidal plants research have gone to the extent of evaluating essential oils from these plant species and determining active compounds against storage pests, our study reports on an innovative approach to the use of plant based pesticides that can readily be implemented in traditional grain protection methods especially by resource poor farmers in sub-Saharan Africa.
Grain
Untreated maize grain was sourced from a single farmer in Mashonaland West Province of Zimbabwe (17°20'51"S 30°12'30" E). In the laboratory, grain was sieved to remove fluffy material and other foreign matter (Masiiwa, 2004). Any hidden infestation in the grain was removed by putting the grain in the oven at 40°C for four hours (Bekele, 2002). Disinfested grain was kept in a freezer at approximately -1°C to prevent further infestation.
Grain treated in this manner was used to rear S. zeamais used in the experiments. For experimental purposes, grain was removed from the freezer and allowed to acclimatize at ambient temperature and relative humidity until it attained a moisture content of 12%. At the prevailing conditions (approximately 26°C and 40% relative humidity), this process took two days. Moisture content, the percent of broken and seed viability were assessed before commencement of experiments.
Plant materials
Fresh and healthy E. grandis leaves were collected from the GMB Aspindale campus (17° 50' S, 31° 03' E.) in Harare, Zimbabwe during the month of August, while T. minuta leaves were gathered from Warren Park herbal garden (17°51'50'' S, 31°1'47'' E) in Harare during the same period. Plant species identification was done before commencement of studies and at the Zimbabwe National Botanic Gardens in Harare, Zimbabwe. Harvested leaves of T. minuta and E. grandis were spread and air dried under shade at room temperature of 27 to 30°C for 10 to 12 days respectively to minimize the degradation of volatile compounds. The dried leaves were ground to powder using a Thomas Wiley® laboratory mill, sieved through a 1.5 mm sieve to obtain a finer powder.
Insects
S. zeamais sourced from a pure colony maintained at the Department of Biological Sciences, University of Zimbabwe was reared on maize under ambient laboratory conditions (approximately 26°C and 40% relative humidity). Six hundred unsexed adult S. zeamais were reared in one-litre glass jars containing 300 g of uninfested maize grains. The top of each glass jar was covered with a cloth and fastened tightly with rubber bands.
Insects were allowed to oviposit for 10 days after which all adult insects were removed through sieving (Tefera et al., 2011). Sieved grain was placed in clean jars and left for a period of 28 to 30 days during which emergence of adults was assessed by sieving the grain. At 27°C and relative humidity (RH) 65 ± 5% S. zeamais completed the life cycle in 28 days (Hill, 1987). Although the prevailing, RH in this study was lower (approximately 40%) adults emerged at approximately 30 ± 2 days.
Emerging adult insects were collected and kept in separate jars according to their age. Adults that emerged on the same day were considered of the same age. New generations were sustained by the replacement of devoured grain with fresh and uninfested grain. Experiments were conducted using the first generation of insects reared on the same maize batch which was also used for experiments.
Grain treatments
Across all the six treatments, 10 kg samples of maize grain were used. The treatments were E. grandis, T. minuta, Actellic Chirindamatura® dust (16 g/kg Pirimiphos-methyl + 3 g/kg Permethrin) at 0.5 g/kg (positive treatment) and an untreated control being the negative control; each replicated three times. The ground leaf powders were applied evenly throughout the grains at two rates (2.5 and 5 g/kg) converting to 25 g/10 kg and 50 g/10 kg respectively. Mixing was done manually. A total of 40 randomly selected insects per 10 kg sample were used as initial infestation.
Grain sample analyses
Initial sub-samples were taken at the beginning of the experiment and subsequent sampling was done at 32 days (approximately one month) intervals for a period of 192 days (approximately six months). All samples of the requisite mass were collected at each sampling time using a sampling spear drawing grain from different positions of each bag. The sub-samples were analyzed in the laboratory to determine grain weight loss (%), number of live insects, seed germination (%), grain colour and odour.
Live infestation
A procedure by Chikukura et al. (2011) with modifications was used to estimate insect infestations in the experiment. One kilogram sub-sample was weighed and sieved trough a 1.5 µm sieve. Live insects were physically counted and recorded after every 32 days for 192 days. A variety of botanical plants or their extracts have been shown to cause a number of insect population depressing effects such as mortality (Wanyika et al., 2009), anti-feeding (Liu et al., 2002), repellence and anti-oviposition (Ukeh and Umoetok, 2011; Ukeh et al., 2011) when applied against storage insect pests. For this study indirect assessments of these effects were assessed by estimating the population of the resultant progeny of infested insects. Under the prevailing experimental conditions (Hill, 1987) estimated a life cycle period of 28 days. Our assessments were therefore done after every 32 days to capture the population of newly emerging adults. Grain samples and insects were returned to the respective treatments after assessments. New independent samples were drawn from respective treatments in subsequent assessments.
Weight loss
Sub-samples were assessed for damage caused by insect infestations every 32 days for 192 days. Two hundred gram sub-samples were weighed using an Adams® scale. The weight and number of undamaged and insect damaged grains were assessed and used to calculate the percentage grain weight loss using the method described by Gwinner et al. (1996).
Weight loss (%) = UNd - DNu × 100/U (Nd + Nu)
Where U = weight of undamaged grains; D = weight of insect damaged grains; Nu = number of undamaged grains and Nd = number of damaged grains.
Seed germination percentage
The effect of treatments and storage duration on seed viability was investigated over a six month grain storage period. An initial sample of 500 g from the undamaged grains was subdivided using a riffle divider and a working sample of 100 g was obtained for the seed germination tests (Chikukura et al., 2011). The sub-samples were germinated on moistened filter paper (Whatman No. 1) in Petri-dishes with three replicates. The germination trays were maintained under laboratory conditions of 27 ± 2°C and approximately 40% relative humidity. The number of emerged seedlings from the trays were counted and recorded after seven days. The percentage germination was computed as follows:
% seed viability = (NG × 100) / TG
Where NG = number of seeds that germinated and TG = total number (=100) of test seeds placed in each tray (Uke et al., 2011).
Grain colour and odour
The change in grain colour and odour of the treated and untreated samples was assessed three times namely at the beginning of the storage period, three and six months after grain treatment. The sub-samples were drawn from treated grain and cleaning by blowing off the residual particles using a fan. Samples were assessed for change in odour and colour by use of a scoring scale of 1 to 5 that was defined separately for each of the two parameters (Ogendo et al., 2004). Scoring for change in grain odour was done according to the following scale: 1: Grain is odourless, 2: Grain has little offensive odour, 3: Grain has moderately offensive odour, 4: Grain has offensive odour, 5: Grain has very offensive odour making it unacceptable for human consumption.
Scoring for change in grain colour was done using a scale of 1 to 5 as follows: 1: No detectable change in colour, 2: Slight change in colour, 3: Moderate change in colour, 4: Great change in colour , 5: Highly significant change making grain unacceptable for human consumption. Each sample was coded and presented in a well-lit and ventilated laboratory room for assessment. A panel consisting of 15 independent assessors scored for change in grain colour and odour (Ogendo et al., 2004). Assessors were allowed into the assessment room, one at a time to ensure independence of scores. Blank scoring sheets were used for each assessment date to ensure that there is no bias due to previous data.
Data analysis
Repeated measurements on total insect count, weight loss (%), and percentage germination were obtained. All the data collected were first homogenized using appropriate logarithmic transformations (Log X + 1.5 for live infestation and insect damage and arcsine X for percent germination) to normalize them before being subjected to analysis of variance (ANOVA) and Generalized Linear Models in SAS statistical software (SAS-2006-2008). Descriptive statistics were used to evaluate grain colour and odour.
Live infestation
Across all the grain treatments, storage duration had significant effect (F5, 72 = 376.20, P<0.05) on the number of live insects in the grain samples. Tagetes minuta applied at a rate of 5 g/kg showed no significant effect (P>0.05) in the number of live insects 96 days (approximately (≈) three months)) after treatment. However, significant increases in insect numbers occurred from 128 to 192 days (≈month four to six) (Figure 1). The highest mean live infestation (24 insects/kg) was recorded 192 days (≈six months) after treatment.
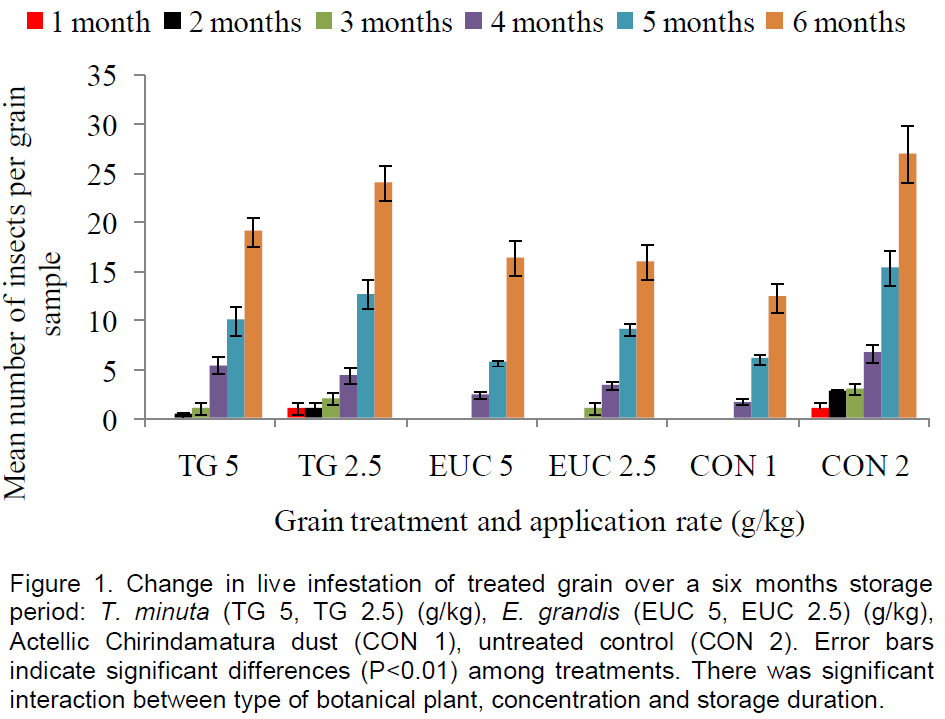
Application of T. minuta at 2.5 g/kg also showed no significant differences 96 days after treatment. An application rate response between 5 and 2.5 g/kg only occurred from 128 days (month four) after treatment, with a rate of 5 g/kg showing significantly (P< 0.05) lower numbers of live insects (Figure 1). At both application rates, that is, 5 and 2.5 g/kg, T. minuta consistently showed significantly higher (P<0.05) number of live insects in the grain samples compared to Actellic Chirindamatura® dust (positive control) for the entire study period. However, compared to the untreated control, T. minuta applications showed significantly lower (P<0.05) live insects in grain samples (Figure 1).
E. grandis applied at a rate of 5 g/kg showed no significant effect (P>0.05) in the number of live insects for the first 96 days after treatment. However, significant increases in insect numbers occurred from 128 to192 days post treatment. The highest mean live infestation (16 insects/kg) was recorded 192 days after treatment. Application of E. grandis at 2.5 g/kg also showed no significant differences 96 days (three months) after treatment. An application rate response between 5 and 2.5 g/kg only occurred from 96 to 160 days (month three to five) after treatment, with a rate of 5 g/kg showing significantly (P< 0.05) lower numbers of live insects (Figure 1). There was no significant (P>0.05) difference in live insects in grain samples after 192 days of application of E. grandis at both application rates.
Compared to Actellic Chirindamatura® dust, E. grandis applied at 5 g/kg showed the same level of control (P>0.05) until 160 days (≈ five months) after application and when applied at 2.5 g/kg, it controlled to the same level until 96 days after application. At both application rates, that is 5 and 2.5 g/kg, E. grandis consistently showed significantly lower (P<0.05) number of live insects in the grain samples for the entire study period compared to the untreated control (Figure 1).
E. grandis applications showed significantly lower (P<0.05) live insects in grain samples compared to T. minuta applications even when applied at half the application rate (Figure 1). However, there was no significant (F25, 72 = 1.29, P = 0.206) interaction between the different grain treatments and storage duration for the live insect infestation.
Weight loss
There was a significant (F6, 84 = 4966.49, P<0.05) decrease in grain weight with storage period across all treatments. The highest mean percent weight loss was 17.5% on the untreated control. There were significant (F5, 84 = 507.68, P<0.05) effects attributed to different forms of grain treatment types and application rates over the 192 days (≈ six months) storage period. There were also significant interaction effects (F30, 84 = 88.29, P<0.05) between grain treatments and storage duration.
After 96 days post treatment, minimal damage was observed across all treatments, however, with a slight increase in the untreated control. From 128 days (≈ four months) onwards, T. minuta applied at 2.5 g/kg had the same level of weight loss as the untreated control, while powders from the same plant cultivar applied at 5 g/kg showed significantly (P<0.05) lower weight loss compared to half the application rate. E. grandis plant powders applied at both 2.5 and 5 g/kg showed significantly lower (P<0.05) weight loss compared to T. minuta application rates and untreated control and to the same low level as the positive control Actellic Chirindamatura ® dust (Figure 2).
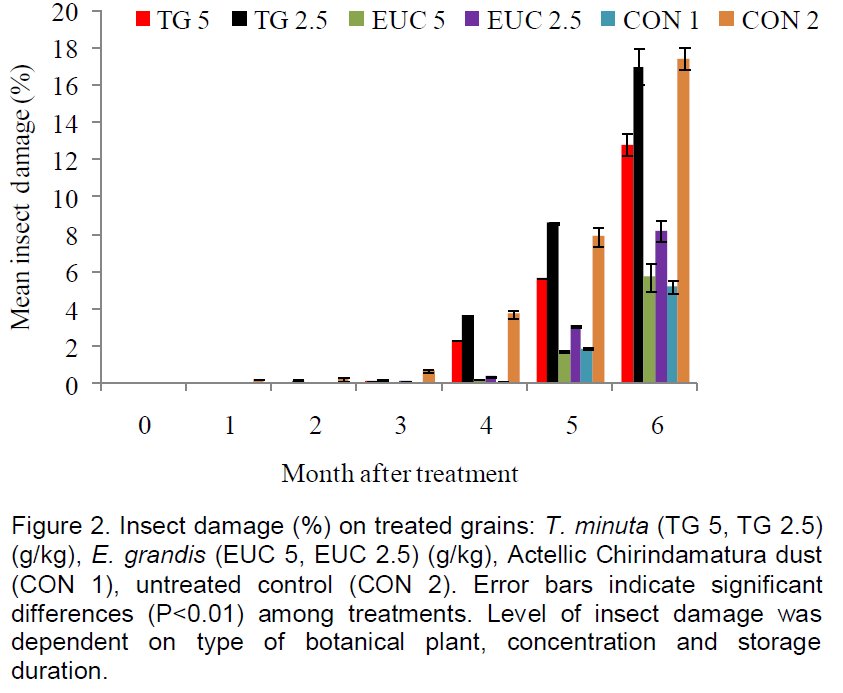
Significantly higher (P<0.05) weight loss were recorded across all treatments from 160 days post treatment up to the end of the experiment (Figure 2). An application rate response was observed for both T. minuta and E. grandis applications. Half the maximum application rates correspondingly showed significantly (P<0.05) higher weight loss in the grain samples (Figure 2). At the end of the experiment, that is, 192 days (≈ six months) of storage, E. grandis leaf powders applied at 5 g/kg maize gave the same level of control as Actellic Chirindamatura ® dust while half this application rate showed significantly lower P<0.05) weight loss compared to T. minuta treatments and untreated control.
Germination percentage
There were significant differences (F2, 36 = 20.09, P = 0.01132) due to the effect of storage duration on the germination percentage of the grains. Treated gain stored for 192 days had generally lower percentage germination across all plant powder treatments. After 96 days of storage, grain treated with T. minuta at 2.5 g/kg, E. grandis 5 g/kg and untreated control showed significantly higher (P<0.05) percent germination compared to the rest of the treatments (Table 1). However, there were no significant treatment (F5, 36 =1.76, P= 0.1468) and storage duration by treatment interaction effects on the percent germination of grains. The mean percent germination across treatments varied from 98% (E. grandis at 2.5 g/kg) to 96.7% (T. minuta 5 g/kg, Actellic Chirindamatura dust and untreated control) (Table 2).
Grain quality parameters
All panelists scored 1 for grain odour at beginning of the experiment indicating that grain were odourless. They
also gave a score of 1 for grain colour indicating uniform grain colour at the beginning of the experiment. After 96 days post treatment, the modal score for colour was 1 (no detectable change in colour) and 1 for odour (grain was oudourless) for grain treated with T. minuta (Table 2). However, grain treated with E. grandis at both application rates had a modal odour score of 2, indicating that grain had little offensive odour. This was the case at both assessments periods (96 and 192 days post application (Table 2).
During the first three months, treatment of grain using plant leaf powders and the recommended insecticide resulted in mortality of live insects found on the grain but failed to kill larvae that were inside grain kernels. This is confirmed by the higher number of insects per grain sample and grain damage in the untreated grain samples compared to those treated with Actellic Chirindamatura ® dust and different plant powder treatment rates.
Several plant powders have been reported to be effective in protecting stored grain products for periods of at most 24 weeks (six months) (Kamanula et al., 2010). Although there was a notable increase in live insect infestation and weight loss of grain treated with plant powders especially for T. minuta, from three to six months after grain treatment, comparison with the untreated grains still indicated that that grain treated with plant powders was better protected against S. zeamais than untreated grain.
E. grandis treatments showed significantly higher levels of efficacy compared to T. minuta treatments at both application rates. Several studies have indicated efficacy of leaf powders and essential oils of most Eucalyptus spp. against many insect species including storage pests (Muzemu et al., 2013; Rajendran and Sriranjini, 2008; Mulungu et al., 2007; Talukder, 2006; Modgil and Samuels, 1998). Eucalyptus spp leaf powders for example, were shown to protect wheat grain against insect pests (Sitophilus oryzae (L.) Coleoptera: Curculionidae), Sitotroga cereallella (Olivier) Lepidoptera: Gelechiidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) for five months while essential oils of Eucalyptus camaldulensis and E. leucoxylon were shown to be lethal to the dates pest carob moth Ectomylois ceratoniae (Zeller) (Lepidoptera: Pyralidae) (Ben-Jemâa et al., 2013; Modgil and Samuels, 1998).
On the other hand, although T. minuta treatments showed less efficacy compared to E. grandis treatments and the positive Atellic Chirindamatura dust, the use of these powders and essential oils and extracts has been recorded to be effective on Callosobruchus maculatus (Fabricius) Coleoptera: Bruchidae) (Shahzadi et al., 2010, Weaver et al., 1994). However, within the scope of the current study, T. minuta applications still showed significant degree of efficacy against S. zeamais compared to untreated control. Plant powders from this plant species can still be used to control S. zeamais where E. grandis is not available.
In the current study, the results of E. grandis powders applied at both 2.5 and 5 g/kg confirm their efficacy in storage pest management and against S. zeamais when compared to the untreated grains and grains treated with a recommended commercial insecticide. This result is of significance in relation to the small-scale farmers throughout the sub-Saharan African region who continue to have problems with grain protection in storage. Subsistence farmers often lack financial resources to purchase recommended pesticides for grain protection. Traditional methods using E. grandis could offer a safer, low cost and more dependable method of maize storage while reducing the need to use excessive amounts of conventional pesticides. However, these results as obtained in this study need to be validated in large scale field studies before they can be widely adopted by farmers.
Farmers often need information on botanicals to support their decision making with respect to reliability of control of particular plant material to reduce insect infestation (Belmain and Stevenson, 2001). Findings from this study give an insight on the degree to which the use of plant powders can readily be used at farm level (effective application rates and expected period of grain protection). This data coupled with further field scale studies can be packaged in the same way conventional pesticides efficacy data is provided.
The efficacy levels and variation over the entire six month grain storage period of E. grandis and T. minuta plant powders observed in this study could be attributed to different plant constituents such as essential oils and alkaloids which impart pesticidal properties to the plants (Manenzhe et al., 2004). It was expected from this study that as storage period of treated grain increased the efficacy of the plant powders also decreased as most the compounds volatilize and degrade from the plant powders as observed by Bekele and Hassanali (2001). The study to a greater extent demonstrated this as more insect infestation and damage was obtained after three months of grain storage.
Seed germination percentage was not significantly affected by plant treatments and the concentration rates. Some small scale farmers in Zimbabwe often use retained seed (stored hybrid and open pollinated varieties) for planting. Seed quality is the prerequisite condition that affects the germination and hence the yield of the crops (Msuya and Stefano, 2010). Some studies have also demonstrated that oils and leaf powders of several plant species have no adverse effects on the germination of maize grain when applied as grain protectants (Manezhe et al., 2004; Ogendo et al., 2004). This attribute of E. grandis and T. minuta is therefore of benefit where retained seed is used and also where germination of grain is required as in the traditional brewing processes.
One of the reported major constraint for widespread use of botanical plants and their essential oils is the effect of residues on food commodities (Rajendran and Sriranjini, 2008). In the current study, grain colour and odour were not significantly altered due to plant powder treatments. These results contradict the general farmer perception that botanical plant powders impart an offensive odour to maize grain (Ogendo, 2000).
Application of T. minuta and E. grandis at 5 g/kg applied to grain did not significantly alter grain colour and odour, which are important parameters in perception or consumer preferences. This could be an indication that constituents from the botanical plant powders were not absorbed by the grains as observed by Jayasekara et al. (2005). However, it should be emphasized that end users of this technology need to remove leaf powders by winnowing to minimize plant powders being incorporated into the maize meal. Grain cleaning by winnowing is a widespread postharvest practice by most smallholder farmers in Zimbabwe. Hence, use of plant powders that requires cleaning before grain is processed for consumption will not result in extra labour input.
Plant powders of E. grandis and T. minuta can be used as natural pesticides in maize storage and can significantly reduce grain damage and live insect infestation with no adverse effects on seed germination, colour and odour. For the purposes of the adoption of this technology, E. grandis should be air dried and ground into powder and admixed with grain at 5 g/kg as a single application at the beginning of the storage season. Protection can be guaranteed for six months. However, for T. minuta application rates of 5 g/kg or more are recommended. The plant materials are effective over a short storage period therefore effective use may be achieved by reapplication of the powders after every three months. E. grandis and T. minuta leaf powders offer promise as alternatives to the synthetic pesticides and may be used to retard the development of insect resistance to widely used conventional insecticides.
The authors have not declared any conflict of interest.
The authors thank the Grain Marketing Board of Zimbabwe (GMB) for providing laboratory facilities for these studies and I. Chidemo (GMB) for assistance in the preparation of laboratory studies. We thank two anonymous referees for their constructive comments.
REFERENCES
|
Adetunji M (2007). Economics of maize storage techniques by farmers in Kwara State, Nigeria. Pakistan J. Soc. Sci. 4:442-450. |
|
|
|
Asawalam EJ, Arukwe UE (2004). Effect of Combination of some plant powders for the control of Sitophilus zeamais. Nigeria Agric. J. 35:76-85. |
|
|
|
Asawalam E, Hassanali A (2006). Constituents of the essential oil of Vernonia amygdalina as maize weevil) protectants. Trop. Subtrop. Agroecosyst. 6:95-102. |
|
|
Bekele AJ (2002). Evaluation of the toxicity potential of Milletia ferruginea (Hochest) Baker against Sitophilus zeamais Motsch. Int. J. Pest Manage. 48:29-32.
Crossref |
|
|
Bekele AJ, Hassanali A (2001). Blend effects in the toxicity of the essential oil constituents of Ocimum kilmandscharicum and O. kanyense on two postharvest insect pests. Phytochemistry 57:385-391.
Crossref |
|
|
Belmain S, Stevenson P (2001). Ethnobotanicals in Ghana: reviving and modernising age-old farmer practice. Pestic. Outlook 12:233-238.
Crossref |
|
|
Benhalima H, Chaundry HMQ, Mills KA, Price NR (2004). Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 40:241-249.
Crossref |
|
|
Ben-Jemâa JM, Haouel S, Khouja ML (2013). Efficacy of Eucalyptus essential oils fumigant control against Ectomyelois ceratoniae (Lepidoptera: Pyralidae) under various space occupation conditions. J. Stored Prod. Res. 53:67-71.
Crossref |
|
|
|
Beti JA, Phillips TW, Smalley EB (1995). Effects of maize weevils (Coleoptera: Curculionidae) on production of aflatoxin B1 by Aspergillus flavus in stored corn. J. Econ. Entomol. 88:1776-82. |
|
|
|
CABI (2012). Sitophilus zeamais data sheet. Last updated. 11 September 2012. Accessed on 18 Novemeber 2014. |
|
|
Caneppele MAB, Caneppele C, Lazzari FA, Lazzari SMN (2003). Correlation between the infestation level of Sitophilus zeamais Motschulsky, 1855 (Coleoptera, Curculionidae) and the quality factors of stored corn, Zea mays L. (Poaceae). Revista Brasileira de Entomologia, Curitiba. 47:625-630.
Crossref |
|
|
|
Chikukura L, Mvumi BM, Chikonzo R, Chenzara C (2011). Evaluation of selected indigenous pesticidal plant powders against stored maize and cowpeas insect pests. Afr. Crop Sci. Conf. Proc. 10:189-192. |
|
|
|
Derera J, Giga DP, Pixley VK (2001). Resistance of maize to the maize weevil: II, Non-preference. Afr. Crop Sci. J. 9:441-450. |
|
|
Dhliwayo T, Pixley KV (2003). Divergent selection for resistance to maize weevil in six maize populations. Crop breeding, genetics and cytology. International Maize and Wheat Improvement Center (CIMMYT), Harare, Zimbabwe. Crop Sci. 43:2043-2049.
Crossref |
|
|
Freer MW, Siebenmorgen TJ, Couvillion RJ, Loewer OJ (1990). Modelling temperature and moisture content changes in bunker-stored rice. Trans. ASAE. 33:211-220.
Crossref |
|
|
|
Fusire M (2008) Integrated Pest Management: Cost-saving techniques for smallholder farmers. Community Technology Development Trust, pp. 1-36. |
|
|
|
Gadzirayi CT, Mutandwa E, Chikuvire TJ (2006). Effectiveness of maize cob powder in controlling weevils in stored maize grain. Afr. Stud. Q. 8:22-26. |
|
|
|
Golop P, Hodges R (1982). Study of an outbreak of Prostephanus truncatus (Horn) in Tanzania. Tropical Product Institute, London, England.16:23. |
|
|
|
Gwinner J, Harnisch R, Muck O (1996). Manual of the prevention of post-harvest grain losses. Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH. P. 338. |
|
|
|
Hill DS (1987). Agricultural insect pest of temperate regions and their control, Cambridge University Press, London P. 659. |
|
|
|
Huang F, Subramanyam B (2007). Effectiveness of spinosad against seven major stored-grain insects of corn. Insect Sci. 14:225-230. |
|
|
|
Jayasekara TK, Stevenson PC, Hall DR, Belmain SR (2005). Effect of volatile constituents from Securidaca longepedunculata on insect pests of stored grain. Chem. Ecol. 31:304-311. |
|
|
Kamanula J, Sileshi GW, Belmain SR, Sola P, Mvumi BM, Nyirenda GKC, Nyirenda SP, Stevenson PC (2010). Farmer's insect pest management practices and pesticidal plant use in the protection of stored maize and beans in Southern Africa: Int. J. Pest Manage. 57:41-49.
Crossref |
|
|
Liu ZL, Xu YJ, Wu J, Goh SH, Ho SH (2002). Feeding deterrents from Dictamnus dasycarpus Turcz against two stored product insects. J. Agric. Food Chem. 50:447-1450.
Crossref |
|
|
Maier DE, Adams WH, Throne JE, Mason LJ (1996). Temperature management of the maize weevil, Sitophilus zeamais Motsch. (Coleoptera: Curculionidae), in three locations in the United States. J. Stored Prod. Res. 32:255-273.
Crossref |
|
|
Manenzhe NJ, Potgieter N, Mabinya LV (2004). Antimicrobial activities of volatile components of Lippia javanica. Phytochemistry 65:2333-2336.
Crossref
|
|
|
|
Mashavakure N (2012). Pest status of the Larger grain borer, Prostephanus trancatus (Horn) (Coleoptera: Bostrichidae) in Zimbabwe and an assessment of the inherent susceptibility of selected maize varieties to the pest. MSc Thesis University of Zimbabwe. |
|
|
Moreno-Martinez E, Jiménez S, Vázquez ME (2000). Effect of Sitophilus zeamais and Aspergillus chevalieri on the oxygen level in maize stored hermetically. J. Stored Prod. Res. 36:25-36.
Crossref |
|
|
|
Modgil R, Samuels R (1998). Efficacy of mint and eucalyptus leaves on the physic-chemical characteristics of stored wheat against insect infestation. Food/Nahrung 42:304-308. |
|
|
Mulungu LS, Lupenza G, Reuben SOWM, Misangu RN (2007). Evaluation of botanical products as stored grain protectant against maize weevil, Sitophilus zeamais (L.), on maize. J. Entomol. 4:258-262.
Crossref |
|
|
|
Mulungu LS, Kubala MT, Mhamphi GG, Misangu R, Mwatawala MW (2010) Efficacy of protectants against maize weevils (Sitophilus zeamais Motschulsky) and the larger grain borer (Prostphanus truncatus Horn) for stored maize. Int. J. Plant Sci. 1:150-154. |
|
|
|
Masiiwa P (2004). Evaluation of the African diatomaceous earths (DEs) as potential maize grain protectants against the maize weevil Sitophilus zeamais. BSc degree honours thesis. University of Zimbabwe. |
|
|
|
Muzemu S, Chitamba J, Mutetwa B (2013). Evaluation of Eucalyptus tereticornis, Tagetes minuta and Carica papaya as stored maize grain protectants against Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Agric. Forest. Fish. 2:196-201. |
|
|
|
Msuya DG, Stefano J (2010). Responses of Maize (Zea mays) Seed germination capacity and vigour to seed selection based on size of cob and selective threshing. World J. Agric. Sci. 6:683-688. |
|
|
|
Nukenine EN, Monglo B, Awasom I, Ngamo LST, Tchuenguem FFN, Ngassoum MB (2002). Farmers' perception on some aspects of maize production, and infestation levels of stored maize by Sitophilus zeamais in the Ngaoundere region of Cameroon. Cam. J. Biol. Biochem. Sci.12:18-30. |
|
|
Oduor GI, Smith SM, Chandi EA, Karanja LW, Agano JO, Moore D (2000). Occurrence of Beauveria bassiana on insect pests of stored maize in Kenya. J. Stored Prod. Res. 36:177-185.
Crossref |
|
|
|
Ogendo JO (2000). Evaluation of insecticidal and repellent properties of Lantana camara and Tephrosia vogelli Hook against the maize grain weevil in maize grain storage in Kenya. J. Food Technol. Afr. 5:29-36. |
|
|
|
Ogendo JO, Deng AL, Belmain SR, Walker DJ, Musandu AAO (2004). Effect of insecticidal plant materials, Lantana camara L. and Tephrosia vogelii Hook, on the quality parameters of stored maize grains. J. Food Technol. Afr. 9:29-36. |
|
|
|
Owusu EO, Osafo WK, Nutsukpu FR (2007). Bioactivities of candlewood solvent extracts against two stored product insects. Afr. J. Sci. Technol. 8:17-21. |
|
|
|
Peng WK, Lee TK, Liao JF (2000). Distribution of phosphine in bins storing bagged sorghum after application of aluminium phosphate. Chin. J. Entomol. 20:45-55. |
|
|
Pereira C, Pereira E, Cordeiro E, Lucia TD, Tótola M, Guedes R (2009). Organophosphates resistance in the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae): magnitude and behavior. Crop Prot. 28:168-173.
Crossref |
|
|
Rajendran S, Sriranjini V (2008). Plant products as fumigants for stored product insect control. J. Stored Prod. Res. 44:126-135.
Crossref |
|
|
|
SAS Institute Inc. (2006-2008). SAS/STAT, Version 9.1. Software Cary, NC, USA. |
|
|
|
Shahzadi I, Hassan A, Khan UW, Shah MM (2010). Evaluating biological activities of the seed extracts from Tagetes minuta L. found in Northern Pakistan. J. Med. Plants Res. 4: 2108-2112. |
|
|
|
Talukder FA (2006). Plant products as potential stored-product insect management agents-A mini review. Emirates J. Agric. Sci. 18:17-32. |
|
|
|
Tefera TM, Mugo S, Ikhayo P, Tende R (2011). Effects of insect population density and storage time on grain damage and weight loss in maize due to the maize weevil Sitophilus zeamais and the larger grainborer Prostephanus truncatus. Afr. J. Agric. Res. 6:2249-2254. |
|
|
|
Thorpe GR (1997). Modelling ecosystems in ventilated conical bottomed farm grain silos. Ecol Modell. 94:255-286;36. |
|
|
Toscano LC, Boica AL Jr, Lara FM, Waquil JM (1999). Resistencia e mecanismos envolvidos em genotipos de milho em relacao ao ataque do gorgulho, Sitophilus zeamais Mots. (Coleoptera: Curculionidae). An. Soc. Entomol. Bras. 28:141-146.
Crossref |
|
|
|
Ukeh DA, Adie EB, Uke JA (2011). Insecticidal and repellent activities of pepper fruit Dennettia tripetala (G. Baker) against the cowpea beetle Callosobruchus maculatus (Fabricius). Biopestic. Int. 7:15-23. |
|
|
Ukeh DA, Umoetok, SBA (2011). Repellent effects of five monoterpenoid odours against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) in Calabar, Nigeria. Crop Prot. 30:1351-1355.
Crossref |
|
|
|
Wanyika HN, Kareru PG, Keriko JM, Gachanja AN, Kenji GM, Mukiira NJ (2009). Contact toxicity of some fixed plant oils and stabilized natural pyrethrum exâ€tracts against adult maize weevils (Sitophilus zeamais Motschulsky). Afr. J. Pharm. Pharacol. 3:066-069. |
|
|
|
Weaver DK, Wells CD, Dunkel FV, Bertsch W, Sing SE, Sriharan S. (1994). Insecticidal activity of floral, foliar, and root extracts of Tagetes minuta (Asterales: Asteraceae) against adult Mexican bean weevils (Coleoptera: Bruchidae). J. Econ. Entomol. 87:1718-1725. |