ABSTRACT
The complete uprooting of diseased mats/fields (CMU) is one of the recommended control options for Xanthomonas wilt of banana. CMU is labour intensive, time consuming and disturbs the soil structure, exposing fields to erosion. CMU often involves exportation of whole plant biomass, affecting soil fertility. The potential of continuous cutting at soil level of all shoots in a mat until complete corm decay in situ as an alternative to CMU was assessed. The first experiment was established using 224 banana mats in their third cropping cycle. All the plants were cut down at soil level, meristems were removed, and sweet potato and bush bean planted. In a repeat experiment with 180 banana mats, a wide range of treatments were applied on top by cutting and removing the apical meristems. These included the: injection of 2,4-D herbicide into the centre of each corm; removal of a cone shaped section from the center of each corm; and creation of a 20 cm deep incision in the center of each corm; in combination with the application of soil or farmyard manure substrate on cut surface. In the first experiment, re-sprouting stopped at 8 months while corms fully decayed after 25 months. Annual intercrops did not influence re-sprouting and corm decay rate. Similar re-sprouting trends occured in the repeat experiment. However, 2,4-D application significantly (P<0.05) lowered decay time, with 12-47% of corms decomposed at 8 months compared with 0-20% in other treatments without 2,4-D. In the 2,4-D treatments, 100% of corms had decomposed compared with 36-80% in other treatments by the 20th month. Deep incisions or cuts did not significantly hasten decomposition. Soil or manure substrate addition had no advantage when compared with the cut surfaces without substrates. A cost-benefit analysis showed a five times higher net income with continuous cutting of re-sprouts when compared with CMU.
Key words: Apical meristems, cost-benefit analysis, decomposition, herbicide, soil, Xanthomonas wilt.
Banana production in the Democratic Republic of Congo (DR Congo) is severely threatened by the new and highly devastating disease, banana bacterial wilt caused by Xanthomonas campestris pv.musacearum (Xcm) (Ndungo et al., 2005). Its non-discriminate infection of all Musa cultivars and ability to cause up to 100% yield loss, severely compromises livelihoods and food security for banana farming households (Tushemereirwe et al., 2003; Ndungo et al., 2005; Karamura et al., 2006; Blomme et al., 2014). It is a vascular disease that results in permanent wilting and eventual death of the banana plant (Yirgou and Bradbury, 1968, 1974). Transmission of this disease is through insects frequently associated with the inflorescence, infected tools, birds, bats, foraging domestic animals and movement of infected plants or plant parts (Biruma et al., 2007; Ocimati et al., 2013; Buregyeya et al., 2014).
The uprooting of diseased mats coupled with banana-free fallows (e.g. grass fallows or cultivation crops such as bush beans, sweet potatos, taro, maize and cassava) has been recommended as a control option for Xanthomonas wilt disease in well-managed banana production systems as in the highland cooking banana (AAA-EA) systems in south-western Uganda and Rwanda (Turyagyenda et al., 2008; Ssekiwoko et al., 2010; Rutikanga et al., 2013; Kubiriba et al., 2014). However, complete mat uprooting is very labour intensive, time consuming and is thus not widely practiced (Jogo et al., 2013; Blomme et al., 2014; Ocimati et al., 2015). In addition, a factor that negatively contributes to the effectiveness of complete mat uprooting is that the majority of farmers do not disinfect their garden tools and yet they often borrow/share tools. They often find disinfection of the garden tools through heating above a fire cumbersome/inconvinient, while chemical disinfection has been perceived by farmers as too expensive or hampered by lack of access (Blomme et al., 2014). Uprooting of diseased mats also results in destruction of the soil structure and exacerbates soil erosion in the affected farms. Coupled to this, the exercise often involves exporting crop debris out of fields, potentially affecting the fertility of the soils.
Herbicides have been used previously to destroy infected banana plants. In Uganda, for example, glyphosate (Roundup®) and 2,4-dichlorophenoxyacetic acid (2,4-D) pseudostem injections have been used to destroy banana plants infected by bacterial wilt in controlled experiments (Okurut et al., 2006; Blomme et al., 2008). Similarly in Martinique, glyphosate has been used to kill off Cavendish plants (Musa AAA group) in preparation for fallow (Quénéhervé, Pers. Comm. In Blomme et al., 2008). Glyphosate has also been used for destroying banana bunchy top infected plants (Musa AAA group) in Hawaii (Sommer, 2000) and Xanthomonas wilt infected plants in Kenya (Kubiriba et al., 2014). Using herbicide injections to destroy bananas has several advantages. First the systemic nature of herbicides means that the whole plant, including the corm, dies and decays in a short time. In contrast, during manual removal or when using a tractor with a set of trailing discs corm pieces can remain in the soil and this could lead to unwanted re-sprouting. The herbicide equipment needed for herbicide application is simple, affordable and requires little skill to operate by farm staff (Blomme et al., 2008). Regarding the cost of 2,4 D, evidence from published research data shows that use of herbicides can be cost-effective and less laborious as compared to physical uprooting of mats, and a small well trained team can eradicate a large acreage in a relatively short time (Blomme et al., 2008). However, some setbacks to the use of herbicides by small-scale farmers in east and central Africa includes their often limited supply, the perceived high cost of herbicides and the possible intake of treated plant material by free-ranging domestic animals (Karamura, personal comm., 2010). Since banana is mainly grown by poor small-scale farmers in east and central Africa (ECA), it is necessary to explore novel environmentally sound methods to destroy infected mats.
The objective of this study was to asses if mat removal through continuous cutting at soil level, of all shoots in a mat could hasten complete corm decay, and thus be a less labour demanding and environmentally sound alternative to manual complete mat uprooting. The effects of two common annual crops, which are often planted after complete mat uprooting to meet household food security needs, on the efficacy of continuous cutting of shoots at soil level were also evaluated.
The first trial was carried out at the “Institut National pour l’Etude et la Recherche Agronomiques (INERA) Mulungu” research station in South Kivu, DR Congo. An existing field with highland cooking banana (AAA-EA; variety ‘Barhabesha’) mats in the 3rd cropping cycle (2nd ratoon cycle) was used. All the plants were cut down during December 2011. All pseudostems in a mat (including peepers, sword suckers and maiden suckers) were cut off with a machete at soil level. In addition, any remaining apical meristem was destroyed/removed using a machete. Two common break crops, namely, the sweet potato, ‘Mugande’ variety and the bush bean, ‘MLB49’ variety, were respectively planted in December 2011 and January 2012 to assess their potential effects on the efficacy of continuous cutting of shoots at soil level.
There were 8 replications per break crop, giving a total of 16 plots. Each plot contained 14 banana mats. Hence, an overall total of 224 mats were assessed. Any re-growth (sprouting suckers) was systematically cut off at soil level, and apical meristems destroyed, at weekly intervals. Corm decay and number of emerging sprouts was assessed at monthly intervals.
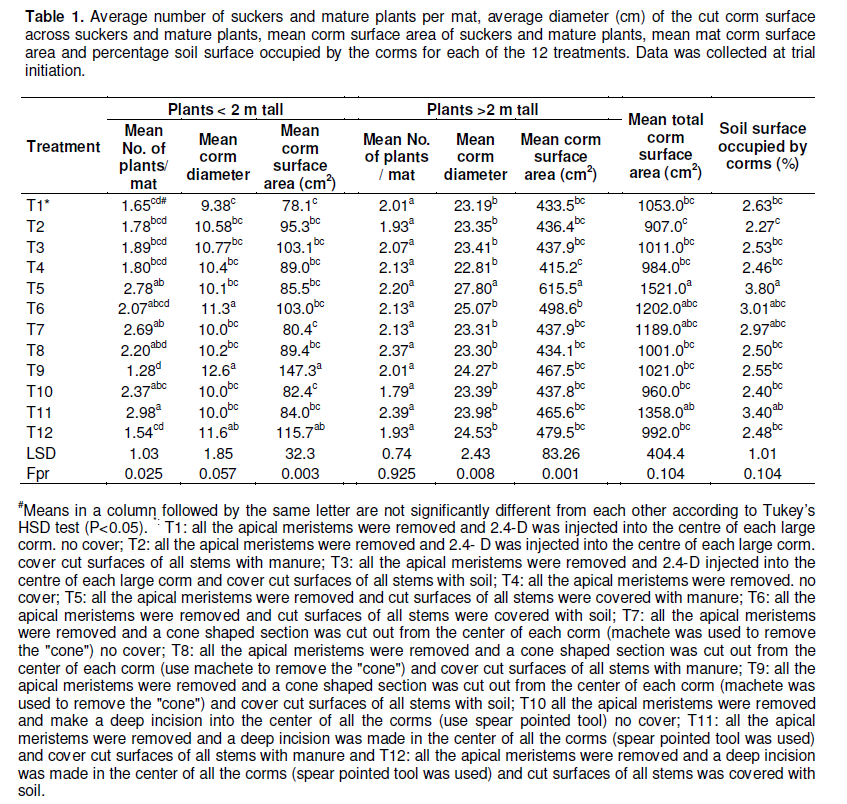
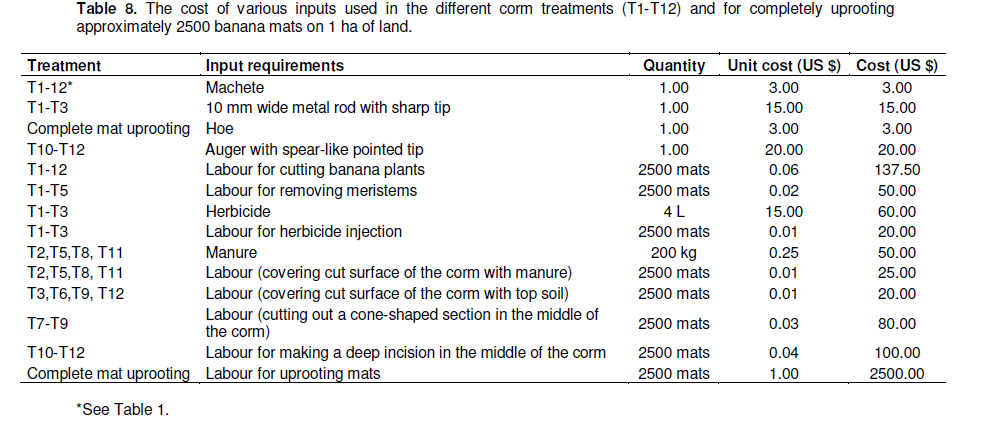
In a second trial, initiated in April 2013, the dessert banana cultivar 'Giant Cavendish' was used. At the initiation of the trial, all pseudostems were cut off at soil level and any remaining apical meristem was removed using a machete. The number of suckers/plants (< 2 meter tall shoots and > 2 m tall shoots) in each mat were counted, cut off at soil level and the diameter (cm) of each plant was measured at the cut-off section (Table 1). These measurements provided information on the approximate mat and corm size and percentage soil surface area occupied by the corms and which was hence not available for annual crop production. In addition, it was hypothesized that the initial variations in total size of corms in a mat could influence its rate of decay. The treatments during this second trial consisted of i) the removal of the apical meristem of all stems ii) the removal of the apical meristem of all stems and the injection of 2,4-D into the centre of each corm, iii) the removal of a cone shaped section in the center of all corms using a machete, and iv) the insertion of a spear like pointed soil auger to at least 20 cm depth into the center of each corm. To ease/facilitate the injection of 1.6 ml of 2,4-D (using a syringe), a 10 mm wide metal rod was inserted up to 10 cm depth, into the corm tissue. In addition to the above treatments, either de-composted manure or top soil was used to cover the cut corm surfaces. Cut surfaces without manure or top soil acted as controls. Treatments iii and iv were anticipated to enhance corm decay as water would stagnate in the cut out corm sections, while micro-organisms from top soil and manure were expected to enhance corm decay. There were 5 mats for each of the 12 treatment combinations and 3 replications, giving a total of 180 mats. Re-sprouts in all the treatments were counted and removed at weekly intervals, while the level of corm and cord root decay was also assessed at monthly intervals.
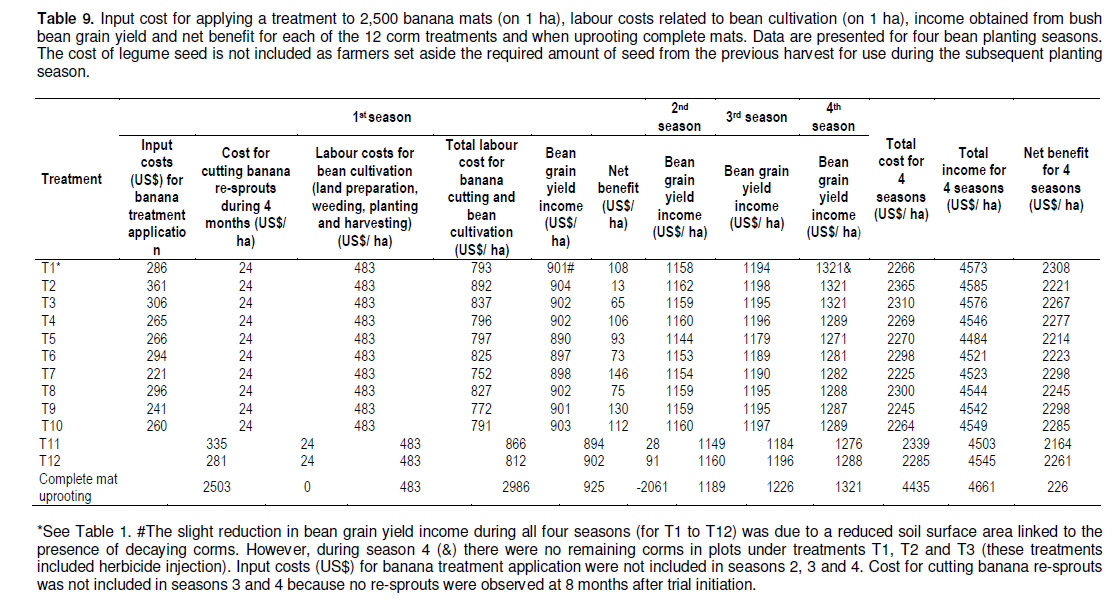
Cord root decay was assessed following a procedure described by Speijer and De Waele (1997) for nematode necrosis assessment. However, instead of digging a 20 x 20 x 20 cm (8,000 cm³) hole, we opted for a smaller 10 x 10 x 10 cm (1,000 cm³) hole in order to minimise possible effects on corm decay. The holes were dug, using a machete, at 20 cm from the mat and all banana cord roots in the hole were collected. All functional/alive roots were counted. Cord root assessment across all treatments began five months after the initiation of the trials (when re-sprouts had stopped emerging) and was carried out monthly from September, 2013 till December, 2013. In addition, further monthly cord root assessments were carried out from May till August 2014. During the initial cord root assessment phase (first four months), all the 15 mats (total of 180 mats) were assessed per treatment while in the subsequent phase, only 6 mats were assessed per treatment (totaling 72 mats). The reduction in mats sampled for cord roots was carried out in order to minimise its potential effect on the rate of corm decay within the mats. Labour costs were computed for the different corm treatments, while 10 mats were completely uprooted in order to calculate the labour costs for manual complete mat uprooting. Corm decay was assessed monthly by slightly cutting the upper (visible) corm tissue using a machete. Only the bush bean ‘CODMLB-001’ variety was planted as a break crop during the second trial as no significant effects of break crop type were observed in the first trial. A cost-benefit analysis was conducted to compare these different treatments and the complete mat removal approach over four seasons of bean cropping.
All data were collated using Excel (Microsoft) and analysis of variance (ANOVA), multivariate analysis of variance (MANOVA) for repeated measurements and means separation with least significant difference at 5% were obtained using the GenStat V. 12 statistical software (VSN International Ltd, 2009). The effect of mat size on the rate of corm decay and total number of resprouts was determined through a simple linear regression of mat size to the percentage of decayed corms at 20 months and the total number of resprouts using the Excel (Microsoft) data anlysis package. Simple linear regression of mat size to corm decay was assessed at 20 months because all corms in the herbicide treatments had already decayed by the 20th month.
Banana corm lateral shoot production and decay under bean and sweet potato break crops
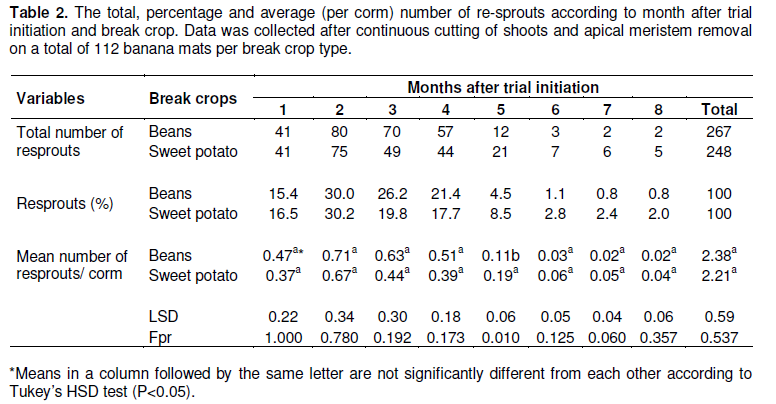
In the first experiment, it was observed that corms continued to produce plantlets until the 8th month (Table 2) from the time the experiments were established. Most of the re-sprouts (varying between 15 and 30% per month) emerged between the first and the fourth months across the break crop treatments. Type of break crop did not significantly (P>0.05) affect re-sprouting (Table 2).
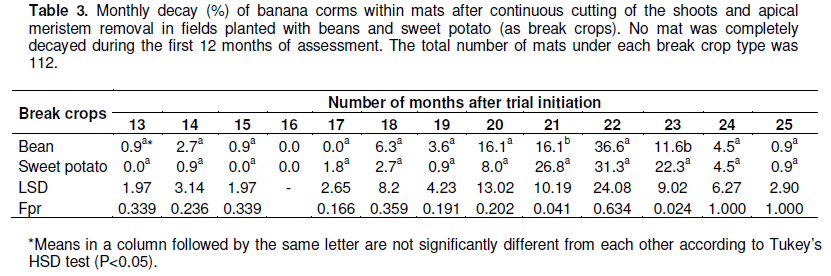
No corm decayed under both bean and sweet potato crop during the first 12 months of the experiment (Table 3). The first decayed mats were noted in the 13th month of the experiment, with most of the corms fully decaying between the 20th and 23rd months of the experiment (Table 3). It took more than 2 years (25 months) for the corms to completely decay. Mat decay was, independent of the presence of the break crops planted in the fields. No significant differences (P >0.05) were observed in corm decay between corms in bean plots and sweet potato plots (Table 3).
In corms used for macropagation, and at the site of this study, shoot emergence was reported to stop between the 7th and 9th months after corm planting in the susbtrate (Ntamwira et al. un-published data), which is in line with the observations made in this study. This could be attributed to nutrient exhaustion in the corms as a result of the continuous removal/cutting of shoots/leaves that are responsible for generating photosynthates under macro-propagation or cutting as in this study. The rate of corm decay has also been reported to be highly correlated with the number of plantlets harvested in macro-propagation experiments (Ntamwira et al., unpublished data). This can be attributed to the fact that the new shoots rely on the stored food reserves in the corm tissues, that over time get exhausted by the continuously removed plantlets. In the absence of fresh assimilates, a rapid degradation of tissues in the corm is expected.
The fact that shoots were produced till the 8th month means that by only repeatedly cutting banana pseudostems at soil level, a common practice observed within communities affected by Xanthomonas wilt, the bacteria Xcm can potentially survive within the corms/farms for at least up to 8 months. However, after 8 month with no additional shoot development and further accumulation of assimilates within the corm tissues to support the survival of the corm tissues, a gradual corm tissue decomposition process is expected, a process unsuitable for the bacteria. Xcm has been reported not to compete effectively with other organisms or survive saprophytically in decomposing plant parts. For example, in studies conducted by Mwebaze et al. (2006), Xcm did not survive in banana plant debris left on the ground surface or buried under the ground beyond 35 days.
Description of mat and corm size in the repeat experiment
The mean number of plants per mat, corm diameter and mat corm surface area of small (< 2 m tall) and big plants (> 2 m tall); mean total corm surface area and percentage surface of soil covered/occupied by corms was only determined for the treatments in the second/repeat experiment (Table 1). Significant differences (P <0.05) were observed in the distribution of small (< 2 m tall) and big (> 2 m tall) plants in terms of the mean surface areas between the different treatments (Table 1). For example, treatment T5 had a larger proportion of its surface area contributed by plants > 2 m tall, while T9 had a higher proportion of small plants (mean surface area of corms) when compared with the other treatments. However, no significant differences (P>0.05) were observed between the treatments for the total mean corm surface areas (sum of mean corm surface area of plants < 2 m tall and plants > 2 m tall) (Table 1). A simple linear regression of the mat sizes to the percentage of decayed corms at 20 months and the total numer of resprouts returned an adjusted R2 value of -0.03 and -0.06, respectively. The F-probability for the regression and P-value for the parameter estimates were also not significant at P<0.05.
These findings suggest that the sizes of the mats did not influence the time to corm decay and the total number of resprouts produced per mat across the treatments. The total percentage soil surface area per hectare occupied by mat corms was limited (less than 3.8%, Table 1) and hence did not affect the available space for annual crop cultivation and the overall profits realized from the cultivation of annual crops.
Effect of additional pseudostem cutting/corm treatments in the destruction of complete banana mats
Re-sprouting in the repeat experiment stopped at 8 months after trial initiation (Table 4). A lower number of lateral shoots (130 to 191) were produced by the corms that had been injected with 2,4-D (T1, T2 and T3) while the highest (314) were produded in treatment T7 (all the apical meristems were removed and a cone shaped section was cut out from the center of each corm (machete was used to remove the "cone") and no cover) (Figure 1 and Table 4). Significant (P< 0.05) effects of 2,4-D on resprouts were observed from the second to the fourth month of experimentation when compared with the treatments without herbicide application (Figure 1A). The observed effect of 2,4-D treatments T1, T2 and T3 can be attributed to the enhanced destruction of corm tissues by the herbicide 2,4-D. For example, 2,4-D had been previously recommended for the destruction of banana mats (Blomme et al., 2008). Addition of manure or soil on the cut corm surface had no significant (P> 0.05) advantage as compared to the control without substrate (Figure 1B). Similarly, additional mechanical damaging of the corm through a cone-shaped cut on the surface or a deep incision in the corm as compared to only cutting of resprouts did not significantly affect the number of resprouts (Table 5).
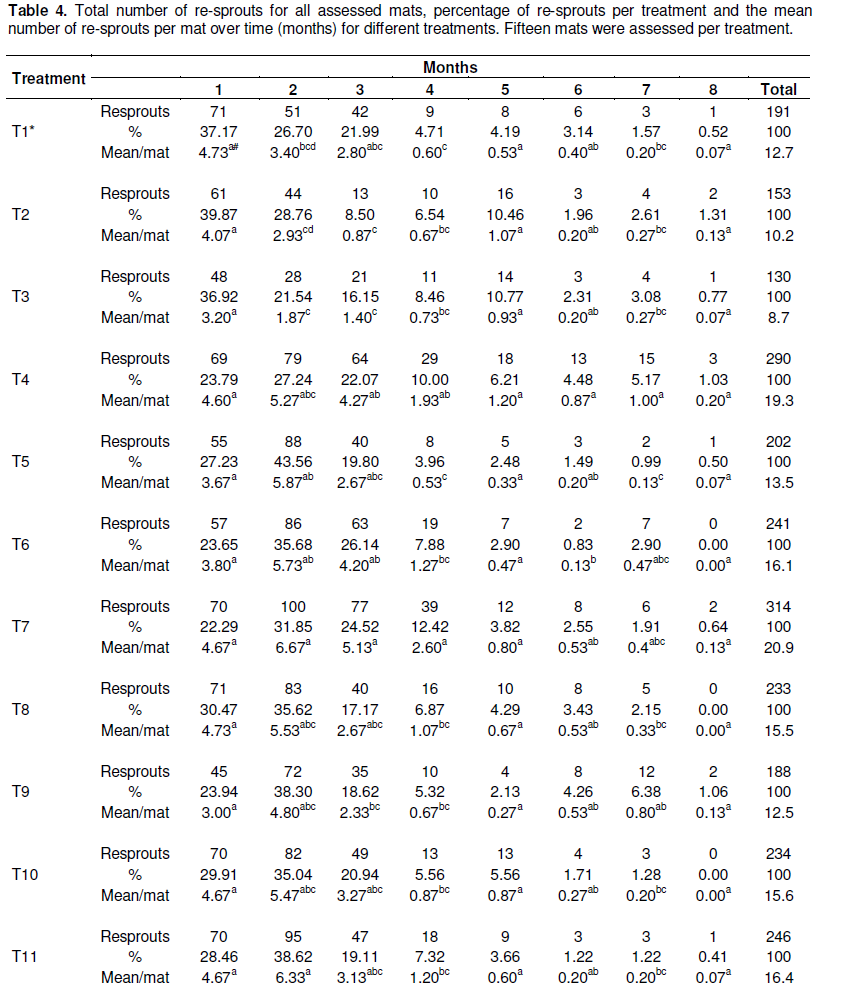
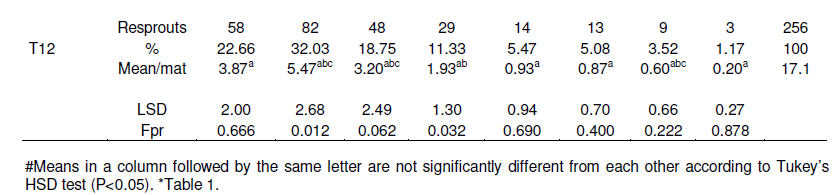
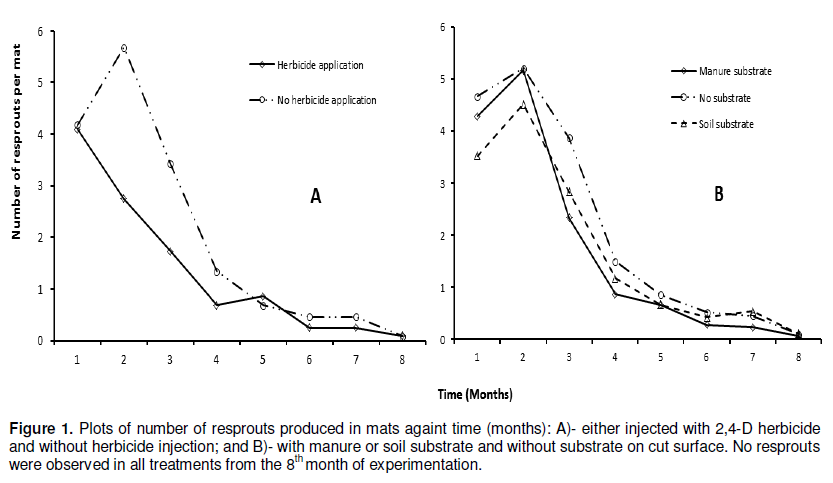
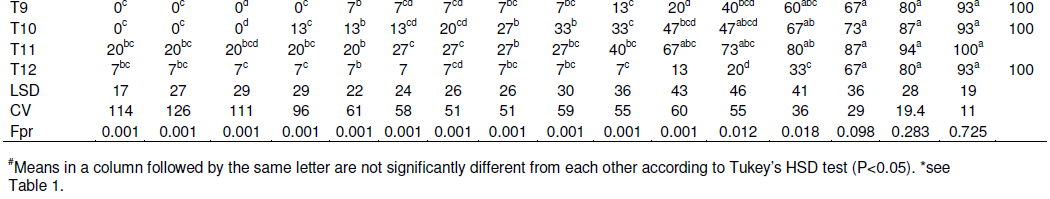
The results of the effectiveness of the different methodsused to destroy banana mats after cutting down the pseudostem at soil level on corm decay are presented in Table 5 and Figure 2. Across all treatments, no single corm completely decayed during the first 7 months after trial initiation. At 8 months, some corms had however completely decayed in five of the 12 treatments, with a significantly higher (47%, P< 0.001) decay in the treatment in which 2,4-D was injected into the center of each corm and the cut stem surfaces covered with manure (T2) (Table 5). Significantly higher (P< 0.001) mat decay was noted in T2 from the 8th to the 14th week, with 93% cumulative mat decay recorded. Herbicide treatment (T2) was followed in effectiveness by treatments T3 (inject cut stems with 2,4-D and cover with top soil) and T1 (inject cut stems with 2,4-D and no cover), respectively. Generally, the three herbicide treatments significantly (P< 0.05) outperformed the other treatments (Figure 2A). For example, at 15 months, about 73 to 93% of the corms under the herbicide treatments had decayed as compared to only 0-27% in the other treatments. Similarly, the herbicide treatments reached 100% corm decay at 20 months, 3 or 4 months earlier than the other treatments (Figure 2A and Table 5).
The deep incision and cone-shaped cut treatments did not significantly vary (P> 0.05) from the control in which re-sprouts were only cut (Table 5). Therefore, an additional effort of making an incision or cut on the corm is not worthwhile.
Mats with corm surfaces covered with manure generally decomposed faster than those without any substrate. However, corm decay rates under the treatments covered with manure or soil did not significantly differ (P> 0.05) from controls that lacked a substrate cover (Figure 2B). Thus, no added advantage with respect to corm decomposition rate is obtained from applying these substrates on cut corm surfaces. However, the 2,4-D treatments, treatment T2 that had decomposed manure applied to the cut surface, recorded significantly higher (P< 0.05) corm decay values relative to the other two treatments T1 (no covering) and T3 (cover with top soil).
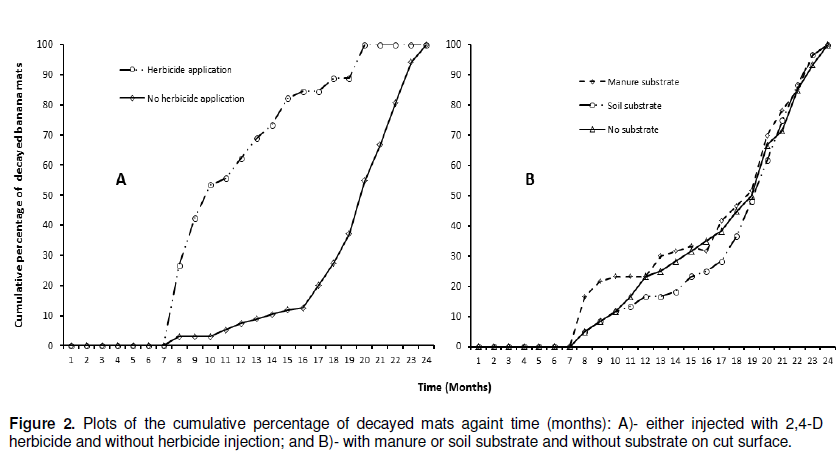
All cord roots had already decayed at 17 months after trial initiation (Table 6). Similar to the number of re-sprouts, a lower number and faster cord root decay was observed for the treatments with 2,4-D injection. The highest number of cord roots (156 roots) were observed for the T7 treatment, while the lowest number of cord roots (51) was observed for the T2 treatment.
.png)
These results indicated that the injection of the herbicide 2,4-D is effective for destroying banana mats. The use of 2,4-D to destroy banana plants/mats has been reported by Sommer (2000), Okurut et al. (2006) andBlomme et al. (2008). Blomme et al. (2008) observed that plants injected with 2,4-D started rotting after three weeks (resulting in snapping of treated plants) and by the 10th week, a complete decay of the treated mats/corms was observed. The same authors also reported that none of the treatments with glyphosate (trade name: Roundup) killed off all the emerging daughter suckers. Although, the sucker leaf lamina edges started showing signs of drying during the first 4 weeks after application (WAA), at 6 WAA, new healthy leaves were formed. In contrast, a 2 ml 2,4-D application killed the emerging daughter suckers. However, at 10 WAA, some re-sprouting from the remaining live parts of daughter sucker corms was still observed. These re-sprouting suckers could however easily be removed from the soil with a knife/machete or even by hand, as they were not firmly attached to the decaying corms of the daughter suckers. Apart from 2,4-D, the application of manure, soil substrate and additional damaging of corms offered no added advantage with respect to the rate of resprouting and corm decay, and is thus not recommended.
Correlation analysis
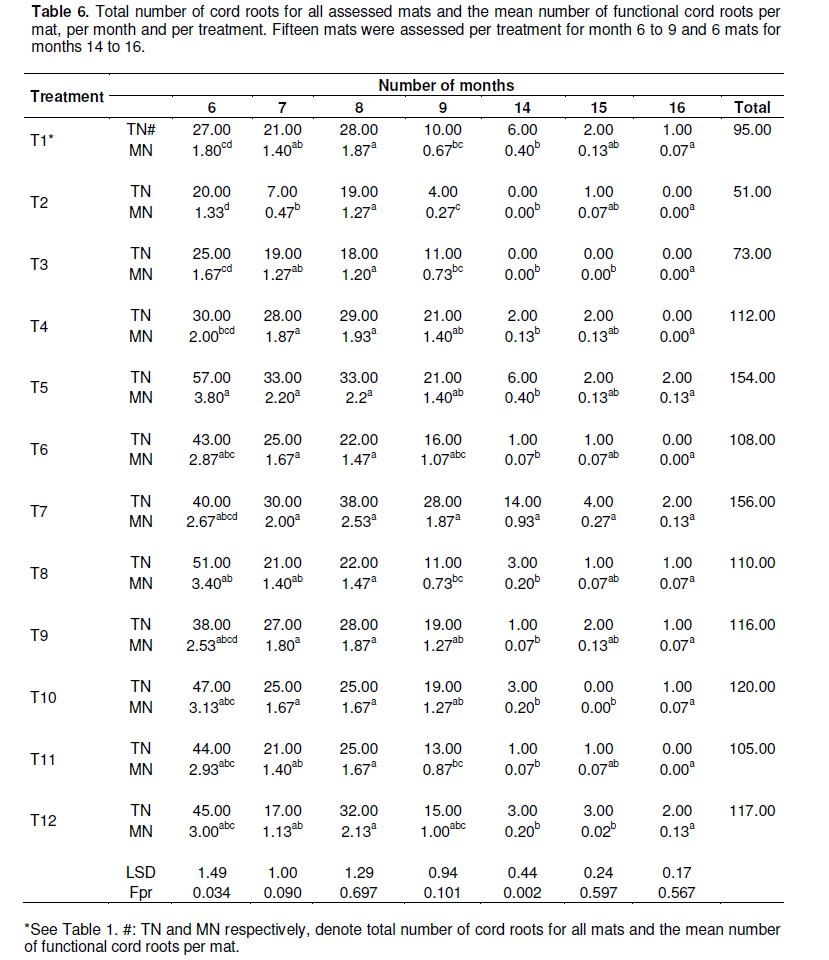
A significant positive correlation was observed between the time to mat decay on one hand and the number of re-sprouts per mat (R2= 0.718) and the number of functional cord roots per mat (R2= 0.801) on the other hand. A larger number of resprouts would drain the corms’ reserves at a faster rate and lead to earlier corm decay as reported by Ntamwira et al. (unpublished data). A higher number of functional cord roots per mat enhances water and nutrient uptake and could hence lead to more vigorous resprouting. This is in agreement with observations made by Sebuwufu et al. (2004) who reported significant positive correlations between shoot and root traits of mats during both the vegetative and reproductive phase for East African highland banana cultivars (Musa AAA-EA genome group). Mean corm diameter was not correlated with other paramaters, while a significant positive relation (R2= 0.670) was observed between the number of functional cord roots and the number of re-sprouts (Table 7).
Cost analysis (labour) comparing complete mat uprooting with continuous stem cutting
The method chosen for removing diseased mats will depend on the resources available to the farmer and the associated net benefits. Uprooting 10 mats (each mat containing an average of 4 plants) takes 4 h for one person at the rate of 10 US$ in the study region. In contrast, the cutting down at soil level of 60 mats (240 plants) takes about 3 h, at a cost of 3.3 US$. The labour cost analysis of the different treatments/methods used in this study showed that the use of these methods can be cost-effective and far less laborious as compared to the physical uprooting of complete mats, and a small well trained team can eradicate a large area in a relatively short time (Tables 8 and 9). The potential net benefit over 4 seasons of bean cultivation (US$/1 ha) that could be obtained after complete corm removal/uprooting was very small (only 226 US$) (Table 9). In constrast, the net benefit for 4 seasons (US$/1 ha) for the T1 to T12 treatments ranged from US$2164 to 2308. This however does not take into perspective the advantages offered by cutting plants to the maintenance or even improvement of the soil structure/fertility conditions, though this may hardly be recognized or considered by farmers.
The results of this study showed that complete corm rotting took more than 2 years, irrespective of break crop type, following the cutting of plants. However, shoot production stopped at 8 months after trial initiation across the different treatments. The cost benefit analysis of this method in comparison with complete mat removal showed that it has 5 times higher net profits. The approach is also relatively easy to apply and corms were also relatively easy to uproot as of 7-9 months after trial initiation due to the absence of new re-sprouts and a limited number of remaining functional cord roots with corresponding poor anchoring of the corms in the soil. This approach is also environmentally sound in the sense that it prevents the drastic destruction of the soil surface associated with digging of corms and the in situ decomposition of roots and corms maintains/enhances soil fertility. Trade-offs between immediate clearing through complete mat rouging for immediate establish-ment of other crops and the soil fertility and labour benefits associated with continuous cutting, however, will need intensive communication to farmers for a wider adoption. It is however not clear if the long survival period of banana corms can support the survival of pathogens (e.g., Xcm or the banana bunchy top virus [BBTV]) previously present in these corms. Disease free fields were used in this study and this was as such not investigated. However, studies have shown that Xcm does not favourably compete under conditions of decomposition as is the case with these corms especially after 8 months when the corm tissues/plant no longer receive assimilates. It is however, recommended to determine the ability of Xcm to survive in such decomposing corm tissues.
The authors have not declared any conflict of interests.
The authors are grateful for the financial support from the Belgian Directorate General for Development (DGD) through the Consortium for Improving Agriculture-based Livelihoods in Central Africa (CIALCA) for the project “Improving agriculture-based livelihoods in Central Africa through sustainably increased system productivity to enhance income, nutrition security, and the environment. This work was carried out in the framework of the CGIAR program on Roots Tubers and Bananas.
REFERENCES
|
Biruma M, Pillay M, Tripathi L, Blomme G, Abele S, Mwangi M, Bandyopadhyay R, Muchunguzi P, Kassim S, Nyine M, Turyagenda L, Eden-Green S (2007. Banana Xanthomonas wilt: a review of the disease, management strategies and future research directions. Afr. J. Biotechnol. 6:953-962.
|
|
|
|
Blomme G, Jacobsen K, Ocimati W, Beed F, Ntamwira J, Sivirihauma C, Ssekiwoko F, Nakato V, Kubiriba J, Tripathi L, Tinzaara W, Mbolela F, Lutete L, Karamura E (2014). Fine-tuning banana Xanthomonas wilt control options over the past decade in East and Central Africa. Eur. J. Plant Pathol. 139:265-281.
Crossref
|
|
|
|
|
Blomme G, Turyagyenda LF, Mukasa H, Eden-green S (2008). The effectiveness of different herbicides in the destruction of banana Xanthomonas wilt infected plants. Afr. Crop Sci. J. 16:103-110.
|
|
|
|
|
Buregyeya H, Kubiriba J, Tusiime G, Kityo R, Ssekiwoko F, Tushemerierwe WK (2014) Role of Birds and Bats in Long Distance Transmission of Banana Bacterial Wilt in Uganda. Int. J. Agric. Innov. Res. 2:2319-1473.
|
|
|
|
|
Jogo W, Karamura E, Tinzaara W, Kubiriba J, Rietveld A (2013). Determinants of farm-level adoption of cultural practices for Banana Xanthomonas wilt control in Uganda. J. Agric. Sci. 5:70-82.
Crossref
|
|
|
|
|
Karamura E, Kayobyo G, Blomme G, Benin S, Eden-Green SJ, Markham R (2006). Impacts of BXW epidemic on the livelihoods of rural communities in Uganda. Proceedings of the 4th International Bacterial Wilt Symposium, 17-20 July 2006, Central Science Laboratory, York, UK. P 57.
|
|
|
|
|
Kubiriba J, Muthomi J, Ndungo V, Kwach J, Erima R, Rwomushana I, Tushemereirwe W, Opio F (2014). Strategies for rehabilitation of banana fields infested with Xanthomonas campestris pv. musacrearum. J. Crop Prot. 3:21-29.
|
|
|
|
|
Kumakech A, Kiggundu A, Okori P (2013). Reaction of Musa balbisiana to banana bacterial wilt infection. Afr. Crop Sci. J. 21:337-346.
|
|
|
|
|
Mwebaze JM, Tusiime G, Tushemereirwe WK, Kubiriba J (2006). The survival of Xanthomonas campestris pv. musacearum in soil and plant debris. Afr. Crop Sci. J. 14:121-128.
|
|
|
|
|
Ndungo V, Eden-Green S, Blomme G, Crozier J, Smith J (2005). Presence of banana xanthomonas wilt (Xanthomonas campestris pv. musacearum) in the Democratic Republic of Congo (DRC). New Dis. Reports 11:18.
|
|
|
|
|
Ocimati W, Nakato GV, Fiaboe KM, Beed F, Blomme G (2015). Incomplete systemic movement of Xanthomonas campestris pv. musacearum and the occurrence of latent infections in xanthomonas wilt-infected banana mats. Plant Pathol. 64:81-90.
Crossref
|
|
|
|
|
Ocimati W, Ssekiwoko F, Buttibwa M, Karamura E, Tinzaara W, Eden-Green S, Blomme G (2013). Systemicity and Speed of Movement of Xanthomonas campestris pv. musacearum in the Banana Plant after Garden Tool-mediated Infection. In Blomme G. van Austen P and Vanlauwe B (eds) Banana Systems in the Humid Highlands of Sub-Saharan Africa: enhancing resilience and productivity CAB International, 2013. pp. 101-108.
|
|
|
|
|
Okurut AW, Tushemereirwe WK, Aritua V, Ragama PE (2006). Use of herbicide for control of banana bacterial wilt in Uganda. Afr. Crop Sci. J. 14:143-149.
|
|
|
|
|
Rutikanga A, Sivirihauma C, Murekezi C, Anuarite U, Ndungo V, Ocimati W, Ntamwira J, Lepoint P, Blomme G (2013). Banana Xanthomonas Wilt Management: Effectiveness of Selective Mat Uprooting Coupled with Control Options for Preventing Disease Transmission. Case Study in Rwanda and Eastern Democratic Republic of Congo. In Blomme G. van Austen P and Vanlauwe B (eds) Banana Systems in the Humid Highlands of Sub-Saharan Africa: enhancing resilience and productivity CAB International. pp. 116-124.
|
|
|
|
|
Sebuwufu G, Rubaihayo PR, Blomme G (2004). Variability in the root system of East African banana genotypes. Afri. Crop Sci. J. 12:85-93.
Crossref
|
|
|
|
|
Sommer A (2000). State prepares to destroy much of Kauai banana crop. Wednesday, May 3, 2000. Honolulu Star-Bulletin.
View
|
|
|
|
|
Speijer PR, De Waele D (1997). Screening of Musa germplasm for resistance and tolerance to nematodes. INIBAP Technical Guidelines 1. INIBAP, Montpellier, France, 42 p.INIBAP Technical Guidelines 1. INIBAP, Montpellier, France 42 p.
|
|
|
|
|
Ssekiwoko F, Turyagyenda LF, Mukasa H, Eden-Green S, Blomme G (2010). Spread of Xanthomonas campestris pv. musacearum in banana (Musa spp.) plants following infection of the male inflorescence. Acta Hortic. 879:349-356.
Crossref
|
|
|
|
|
Turyagyenda LF, Blomme G, Ssekiwoko F, Karamura E, Mpiira S, Eden-Green S (2008). Rehabilitation of banana farms destroyed by Xanthomonas wilt in Uganda. J. Appl. Biosci. 8:230-235.
|
|
|
|
|
Tushemereirwe W, Kangire A, Smith J, Ssekiwoko F, Nakyanzi M, Kataama D, Musiitwa C, Karyaija R (2003). An outbreak of bacterial wilt on banana in Uganda. InfoMusa 12(2):6-8.
|
|
|
|
|
VSN International Ltd (2009). GenStat 12th Edition. www.vsni.co.uk
|
|
|
|
|
Yirgou D, Bradbury JF (1968). Bacterial wilt of Enset (Ensete ventricosum) incited by Xanthomonas musacearum sp.n. Phytopathology 58:111-112.
|
|
|
|
|
Yirgou D, Bradbury JF (1974). A note on wilt of banana caused by the Enset wilt organism Xanthomonas musacearum. East Afr. Agric. For. J. 40:111-114.
|
|




.png)





