ABSTRACT
The root-knot nematode is a major problem for commercial lettuce cultivation because it affects the market quality of the final product. The objective of this study was to select strains of butter-leaf lettuce resistant to Meloidogyne incognita spp. with good agronomic performance for further cultivar trials. Three field experiments were conducted on the premises of Instituto Agronômico de Pernambuco (IPA-PE) and the Federal Rural University of Pernambuco. The experiments were held from October to November, 2012 and March to April, 2013, respectively. In experiment 1, 14 genotypes were evaluated. In experiment 2, 13 genotypes were evaluated. In Experiment 3, 24 progenies were evaluated. In Experiments 1 and 2, commercial characteristics were evaluated. In experiment 3, resistance to M. incognita spp. was evaluated using the following characteristics: Gall index, number of galls and egg number. All of the tested progeny were considered commercial; when comparing the average number of root galls and number of eggs in the root system, 16 and 22 progenies, respectively, were considered resistant. Among all progeny, 13 were shown to satisfy the commercial standard and have significant resistance to M. incognita. Genotypes were selected that had resistance to M. icognita spp. and high commercial potential for further cultivar trials.
Key words: Lactuca sativa L., plant breeding, strain selection, root-knot nematodes.
Lettuce is a vegetable often affected by nematode galls induced by the root-knot nematode (Meloidogyne spp.). Galls form on roots of infected plants, and consequently block the plant’s uptake of water and nutrients from the soil. This prevents normal development of the lettuce head and results in reduced commercial value (Charchar and Moita, 1996). Nematodes are sometimes chemically controlled; however, these products are toxic and often have a residual effect. Therefore, growing nematode resistant cultivars is a more effective form of control.
Inheritance studies done with crossings of the cultivars Regina-71 (susceptible, flat-leaved) and Grand rapids (resistant, curly-leaved) showed that resistance for both M. incognita (Gomes et al., 2000) and M. javanica (Maluf et al., 2002) is controlled by a single genetic locus. Carvalho Filho et al. (2011a) also found that resistance to the M. incognita race 1 in the cultivars Grand Rapids and Salinas-88 is controlled by alleles located in different loci with independent segregation, and the loci which confers resistance in Salinas-88 was assigned the name Me2. Despite the monogenic inheritance, this characteristic is strongly influenced by the environment and in the case of 'Salinas 88', there are still other modifiers (Carvalho Filho et al., 2008).
The monogenic inheritance for this trait is reinforced by studies involving segregating populations. From the crossing of 'Regina-71' and 'Grand Rapids', 10 F2:3 lettuce progenies homozygous for resistance were selected (Fiorini et al., 2007).
In another study involving 'Regina-71' and 'Salinas-88' (resistant parent), 12 F4 progenies homozygous for resistance were selected (Carvalho Filho et al., 2007). When comparing 11 advanced lines of lettuce [(‘Regina-71’ × ‘Grand Rapids’) × ‘Elisa’], six lines were found to be homozygously resistant to M. javanica (Ferreira et al., 2011). These results demonstrate the possibility for selection of resistant homozygous families from crossing contrasting parents.
Studies show that by crossing cultivars with genetic traits of interest, promising offspring can be selected for use as a source of favorable alleles, or perhaps, even be released as a new cultivar (Diamante et al., 2013). Carvalho Filho et al. (2011b) selected heat tolerant progenies when they crossed the Salinas × Grand rapids cultivars. Ferreira et al. (2011), working with advanced lines originating from a cross between Grand rapids and Regina-71, achieved lines resistant to root-knot nematode.
Thus, the objective of this study was to select offspring of butter head-type lettuce crosses resistant to M. incognita spp. for cultivar testing.
Three experiments were conducted to evaluate the performance of various progenies of advanced lettuce crosses. The first and second experiment were performed at two different times at the Agronomic Institute of Pernambuco - IPA, located in Vitória de Santo Antão, Pernambuco state, Brazil (08° 08’ 00’’ S, 35° 22’ 00’’ W; 146 m altitude; 19°C annual temperature). The first was performed from October to November, 2012 (minimum temperature of 20.6°C, and maximum of 36°C), and the second from March to April, 2013 (minimum temperature of 23.3°C and maximum of 34.8°C). The third experiment was conducted in the Greenhouse and Nematology Laboratory of the Plant Pathology Department, Federal Rural University of Pernambuco, Recife, Pernambuco, Brazil (8° 04' 03" S, 34° 55' 00" W; 4 m average altitude; 19°C average temperature) from March to April, 2013.
In experiment 1, five F3:4 strains, five F3:5 strains, two commercial cultivars - Elisa and Regiane, and two advanced lines of these – strains 41 and 62, were evaluated (Souza et al., 2008). The F3:4 strains used in Experiment 1 were obtained according to the genealogy described by Ferreira et al. (2011). The F3:4 offspring were self-fertilized, and the F3:5 progeny were also used in this experiment.
The lettuce seeds were sown in September, 2012, in 128-celled polystyrene trays filled with a commercial substrate. Two to three seeds were planted in each cell. After development of the first true leaves, the plants were thinned to only one plant per cell. Transplanting occurred thirty days after sowing. Three plots measuring 14 m × 1 m in a randomized block design with three replications were used. Each plot was considered a block, and cultivation practices were performed as recommended for the region (Souza et al., 2008). Fertilization was performed as recommended by Cavalcanti (1998) and 15 days after fertilization, mulching with castor bean pomace was performed. Weeding was done when necessary.
The plants were assessed 30 days after transplanting and the variables analyzed were: plant diameter (distance between the edges of the plant in cm), plant height (measured with a ruler at ground level), number of leaves (leaves that were 3 cm above the basal leaves), fresh plant mass (measured from ground level and after cutting off the noncommercial basal leaves), fresh weight of leaves (in kilograms, obtained after counting the total number of leaves), stem length (in centimeters, obtained after counting the number of leaves), stem diameter (measured with calipers), and according to the methodology proposed by Fiorini et al. (2005), the shape of the leaf blade (scores from 1 = wrinkled, to 5 = flat), form of the leaf edge (scores from 1 = cut edge, to 5 = smooth edge), and leaf color (scores from 1 = dark green, to 3 = light green).
In experiment 2, progenies of experiment 1 that stood out as ideal were selected, and so five F4:5 and six F5:6 offspring, together with the cultivars Regiane and Elisa, were used. The experiment employed a randomized design with three replications. Each plot was considered a block with dimensions of 12 m × 1 m. The same variables previously described were again evaluated.
In experiment 3, 24 progenies were evaluated for resistance to M. incognita spp.; seven F5:6 and 17 F4:5 offspring and the two parental cultivars, Grand rapids (resistant) and Regina-71 (susceptible). The seeding was done in polypropylene trays containing a commercial substrate. Fifteen days after transplanting, inoculation with M. incognita eggs was done at a concentration of 800 eggs cell-1, corresponding to 20 eggs cm-3. The inoculation, as well as the extraction of the eggs, was made ​​according to the methodology of Hussey and Barker (1973), as modified by Boneti and Ferraz (1981). A randomized block design was used with three replications of eight plants per plot.
Evaluations were performed 45 days after inoculation, using test plants to verify the efficiency of the inoculum. Number of galls with substrate, number of galls without substrate and egg numbers, was evaluated. For the number of galls with substrate (gall index), a rating scale of 1 to 5 was used and based on the following criteria: 1 = few visible (< 10 galls) and small galls (< 1 mm); 2 = few visible galls, but intermediate in size (1 to 3 mm); 3 = intermediate number of galls visible (10 to 30 galls), with few large galls (> 3 mm); 4 = many visible galls (< 30 galls), predominantly large (> 3 mm), with few coalesced galls; and 5 = large and visible galls (> 30 galls), many already coalesced (Fiorini, 2007). For number of galls without substrate (total number of galls), the roots were washed, avoiding a direct water jet on the roots, and the galls were counted. Finally, the number of eggs was accessed. For this, the egg extractions were performed according to the technique of Hussey and Barker (1973) and modified by Boneti and Ferraz (1981). A Motic® BA310 Optical System stereoscopic microscope was used for counting the eggs.
Analyses were performed using the SAS program (SAS Institute, Inc., 2002). Prior analyses of the data found non-normality and the means were transformed by applying the square root methodology according to Box and Cox (1964). The averages of the Grand rapids (resistant) and Regina-71 (susceptible) cultivars were evaluated, comparing them separately to the averages of each offspring using the Dunnett test (P < 0.05). The lack of significance between the offspring and the cultivar Grand rapids (resistant) and the significance in relation to Regina-71 (susceptible) for all characteristics, identify the offspring as homozygous resistant. On the other hand, no significant difference between the progeny and the cultivar Regina-71 (susceptible), and the significance in relation to the cultivar Grand Rapids (resistant) for all characteristics, identify those offspring as susceptible. Other observed situations were still considered segregating. For experiments 1 and 2, analyses of variance were carried out and significance after the F-test means were obtained and grouped by Scoot-Knott test (P < 0.05) and analyzed with the aid of the GENES program (Cruz, 2013).
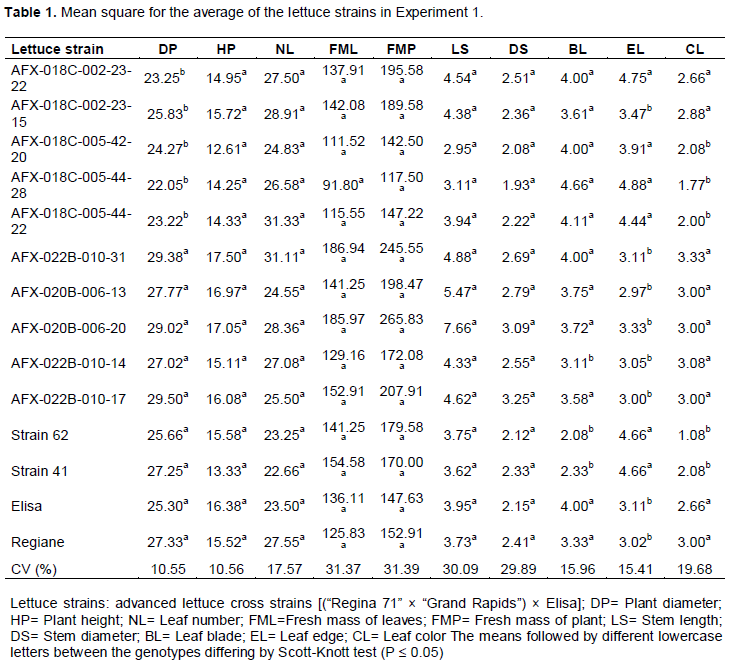
There were significant differences for the traits of plant diameter, shape of leaf blade, shape of leaf edge and leaf color when the F3:4 and F3:5 progenies were analyzed (Table 1). For plant diameter, the progenies formed two groups; one formed by the F3:5 offspring and a second group which included both the F3:4 offspring and the commercial control strains 62 and 41. The averages of these two groups ranged from 22.05 to 25.83 cm for the first group and from 25.30 to 29.38 cm for the second group. These results qualify the F3:4 progeny for further testing in order to obtain more productive strains. In addition, values ​​for plant diameters ranging from 10.7 to 16.6 cm have been found in the literature (Souza et al., 2008), so according to the classification of Zárete et al., (2010), all progenies tested were considered commercial, as they had a diameter greater than 20 cm. We highlight the progeny AFX-022B-010-17, with an average diameter of 29.50 cm, outperforming all commercial lines and the commercial controls.
There was no significant difference between the means of the genotypes and the cultivar Elisa, thus, these genotypes were classified as flat- leaf blade. However, there was significant difference among the evaluated commercial controls and the strains 41 and 62, which had wrinkled leaf blades (Table 1). In addition, for the characteristic of leaf color, strains 41 and 62 differed from the controls, as well as the F3:4 progeny and the two F3:5 progenies, AFX-018C-002-23-22 and AFX-018C-002-23-15 (Table 1). This is mainly due to the selected goal that was carried out for each line of research (Carvalho Filho et al., 2009). This fact is important because it emphasizes the efficiency of selection for color and shape of the leaf blade. Only four strains; AFX-022B-010-14, AFX-018C-005-42-20, AFX-018C-005-44-28, and AFX-018C-005-44-22, did not have leaf blade form and color similar to the respective cultivar, and thus were considered segregating for these characteristics. Phenotypic segregation was observed when evaluating advanced lines and transgressive segregation towards wrinkling leaf blades (Fiorini et al., 2005; Silva et al., 2008). These results show that for these characteristics, the genetic control is found on more than one gene, and these genotypes can be recovered through backcrossing in later generations.
For the formation of the leaf edges, there was significant difference between progenies and a special significant difference between the controls, Elisa and Regiane, and the strains 41 and 62 (Table 1). For this characteristic, the strains 41 and 62 received the highest scores, having a smoother edge than the cultivars. Thus, the group with leaves having smoother edges included: AFX-018C-002-23-22, AFX-018C-005-42-20, AFX-018C-005-44-28, and AFX-018C-005-44-22, and in the group with more jagged or cut edges, the progenies were: AFX-018C-002-23-15, AFX-022B-010-31, AFX-020B-006-13, AFX-020B-006-20, AFX-022B-010-14, and AFX-022B-010-17. For the trait of leaf-edge shape, Silva et al. (2008) found segregation between and within lineages and transgressive segregation. There was no significant difference between plant height, number of leaves, fresh weight of leaves, fresh weight of plant, stem length and stem diameter. The variation coefficients ranged from 10.56% for the trait “plant height” to 31.39% for “fresh weight of plant”, showing the efficiency of the experimental design.
.png)
For the characteristics viz., number of leaves, fresh weight of leaves and weight of the plant, which all have high agronomic importance, the progenies did not differ from commercial cultivars. For the number of leaves, these genotypes obtained approximate values of 28, 31, 24, 28, and 25 leaves head-1, respectively. Similar values ​​were found by Ferreira et al. (2013), with an average of 26 leaves head-1. The fresh weight of plant values ​​ranged from 142.50 to 265.83 g plant-1, exceeding strains 41 and 62. So, considering all evaluated characters, the offspring selected for evaluation in experiment 2 were: AFX-18C-002-23-15, AFX-020B-006-13 and AFX-022C-010-17.
There were no significant differences for the traits of plant diameter, plant height, fresh weight of plant, stem length, stem diameter and leaf border shape compared to the commercial controls and among the progeny (Table 2). The progenies obtained higher means when compared to the commercial control (Diamante et al., 2013). Similar behavior was observed in both studies, and therefore shows that the majority of loci genes are fixed for these characteristics.
The leaf number trait is indicative of the genetic material’s environmental and commercial adaption commercialization (Diamante et al. 2013), and improvement of this trait is of paramount importance for lettuce, since its leaves are the commercial end product. There were differences between the progenies; only six of the 11 evaluated progenies were significant underperformers when compared to the other progenies and the cultivars (Table 2).
For resistance to M. incognita spp., the cultivars Grand rapids and Regina-71 showed lower and upper limits, with respective averages of 1.64 and 4.48, thereby proving their resistance or susceptibility based on the visual gall index (Table 3). From a total of 24 progenies, twelve progenies differed significantly from cultivar Regina-71, but did not differ from cultivar Grand rapids, and were considered resistant based on the visual gall index. On the other hand, an equal number showed the reverse difference, and therefore, were considered segregating. When comparing the average number of root galls, only eight progeny (AFX-018D-02-23-15-05, AFX-018D-02-23-15-08, AFX-022C-10-31-05, AFX-022C-10-31-11, AFX-020C-06-13-02, AFX-020C-06-13-05, AFX-020C-06-13-06, and AFX-022C-10-17-01) did not differ for both cultivars, and were considered segregating. Sixteen progeny were considered resistant for this character (Table 4). For the average number of eggs in the root system, 22 of the 24 progenies were considered resistant to that variable and two progenies (AFX-022C-10-31-12, AFX-022C-10-17-01) were considered segregating (Table 5).
Among all progenies, 13 were shown to satisfy both the commercial standard and showed significant resistance to M. incognita spp.. Furthermore, the progenies AFX-020C-06-13-02, AFX-020C-06-20-03, AFX-020C-06-20-02, and AFX-020C-06-20-04 originated from families considered segregating for M. javanica (Ferreira et al., 2011). In addition, the same behavior for resistance was found by Gomes et al. (2000) in a mixture of progenies from these families when tested in F4 and F5 for resistance to M incognita spp.. Finally, Ferreira et al. (2011) evaluated these progenies for M. javanica resistance and found six resistant progenies, suggesting that maybe the control is found on the same locus.
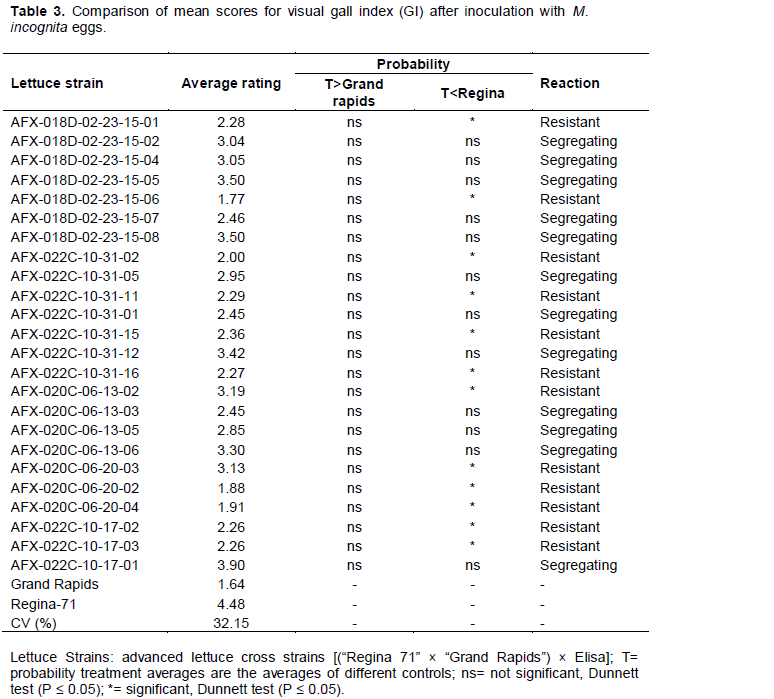
In this work, 13 progenies were found to be resistant for all the evaluated M. incognita spp. traits. Therefore, these results confirm the hypothesis that the same locus controls resistance to M. javanica and M. incognita spp. However, these progenies need to be evaluated for races 1, 2, 3 and 4 to confirm these results, because the assessments made in this work, and also by Gomes et al. (2000), were done with a mixture of races without knowing for certain what races were involved.
With regard to the visual gall index (Ferreira et al. (2011), this trait is of paramount importance for the genetic improvement program, as it directly affects the lettuce seedling’s health. Resistance for this trait permits a continuation of the program, because it allows a plant to grow and produce seed. In general, for both commercial characteristics and resistance, the progeny AFX-018D-02-23-15-01 stood-out for further cultivar testing.
The authors have not declared any conflict of interests.
The authors thank the Foundation for Science and Technology of Pernambuco (FACEPE) and the Agronomic Institute of Pernambuco (IPA) for granting a Masters scholarship and providing the area for production and installation of the experiments.
REFERENCES
|
Boneti JIS, Ferraz S (1981). Modificação do método de Hussey & Barker para extração de ovos de Meloidogyne exigua de raízes de cafeeiro. Fitopatol. Bras. 6:553.
|
|
|
|
Box GEP, Cox DR (1964). An analysis of transformations. J. R. Stat. Soc. 26:211-252.
|
|
|
|
|
Carvalho Filho JLS, Gomes LAA, Maluf WR (2009). Tolerância ao florescimento precoce e características comerciais de progênies F4 de alface do cruzamento Regina 71 x Salinas 88. Acta Sci. Agron. 31:37-42.
Crossref
|
|
|
|
|
Carvalho Filho JLS, Gomes LAA, Maluf WR, Oliveira RR, Costa DS, Fereira S, Monteiro AB, Costa RR (2011b). Resistance to Meloidogyne incognita race 1 in the lettuce cultivars Grand Rapids and Salinas-88. Euphytica 182:199-208.
Crossref
|
|
|
|
|
Carvalho Filho JLS, Gomes LAA, Silva RR, Ferreira S, Carvalho RRC, Maluf WR (2011b). Parâmetros populacionais e correlação entre características de resistência a nematoides de galha em alface. Rev. Bras. Ciênc. Agrárias 6:46-51.
|
|
|
|
|
Carvalho Filho JLS, Gomes LAA, Westerich JN, Maluf WR, Campos VP (2007). Caracterização de famílias F4 de alface de folhas lisas quanto à homozigose para resistência à Meloidogyne incognita. Rev. Bras. Agrociência 13:331-336.
|
|
|
|
|
Carvalho Filho JLS, Gomes LAA, Westerich JN, Maluf WR, Campos VP, Ferreira S (2008). Inheritance of resistance of 'Salinas 88' lettuce to the root-knot nematode Meloidogyne incognita (Kofoid & White) Chitwood. Curr. Agric. Sci. Tech. 14:279-289.
|
|
|
|
|
Cavalcanti FJAC (1998). Recomendação de adubação para o estado de Pernambuco 2º aproximação. 2. IPA, Recife, Pernambuco, Brasil.
|
|
|
|
|
Charchar JM, Moita AW (1996). Reação de cultivares de alface à infecção por mistura populacionais de Meloidogyne incognita em condições de campo. Hortic. Bras. 14:185-189.
|
|
|
|
|
Cruz CD (2013) GENES - a software package for analysis in experimental statistics and quantitative genetics. Acta Sci. 35:271-276.
|
|
|
|
|
Diamante MS, Seabra JS, Inagaki AM, Silva MBD, Dallacort R (2013). Production and resistance to bolting of loose-leaf lettuce grown in different environments. Revista Ciência Agronômica 44:133-140.
Crossref
|
|
|
|
|
Ferreira LL, Aniceto RR, Ribeiro TDS, Almeida DG, Porto VCN (2013). Comportamento de variedades de alface na semeadura de março no município de Areia-PB. Sci. Plena 9:1-7.
|
|
|
|
|
Ferreira S, Vieira VLF, Gomes LAA, Maluf WR, Carvalho Filho JLS (2011). Identificação de linhagens avançadas de alface quanto à resistência a Meloidogyne javanica. Ciência e Agrotecnologia 35:270-277.
Crossref
|
|
|
|
|
Fiorini CV, Gomes LAA, Maluf WR, Fiorini IV, Duarte RDP, Licursi V (2005). Evaluation of the F2 generations of lettuce for resistance to root-knot nematodes and tolerance to early bolting. Hortic. Bras. 23:299-302.
Crossref
|
|
|
|
|
Fiorini CVA, Gomes LAA, Libânio RA, Maluf WR, Campos VP, Licursi V, Moretto P, L. Souza AD, Fiorini IVA (2007). Identification of the F2:3 homozigotic lettuce families resistant to the root-knot nematode. Hortic. Bras. 25:509-513.
Crossref
|
|
|
|
|
Gomes LAA, Maluf WR, Campos VP (2000). Inheritance of the resistant reaction of the lettuce cultivar 'Grand Rapids' to the southern root- knot nematode Meloidogyne incognita (Kofoid & White) Chitwood. Euphytica 114:37-46.
Crossref
|
|
|
|
|
Hussey RS, Barker KRA (1973). Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Report. 57:1025-1028.
|
|
|
|
|
Maluf WR, Azevedo SM, Gomes LAA, Oliveira A (2002). Inheritance of resistance to the root-knot nematode Meloidogyne javanica in lettuce. Genet. Mol. Res. 1:64-71.
Crossref
|
|
|
|
|
SAS Institute (2002). Getting started with the SAS learning edition 2000. p. SAS Institute, Cary, North Carolina, USA.
|
|
|
|
|
Silva RR, Gomes LAA, Monteiro AB, Maluf WR, Carvalho Filho JLS, Massaroto JA (2008). Linhagens de alface-crespa para o verão resistentes ao Meloidogyne javanica e ao vírus mosaico-da-alface. Pesqui. Agropecu. Bras. 43:1349-1356.
Crossref
|
|
|
|
|
Souza MDC, Resende LV, Menezes D, Loges V, Souto TA, Santos VFD (2008). Genetic variability for agronomic characteristics in lettuce progenies with heat tolerance. Hortic. Bras. 26:354-358.
Crossref
|
|
|
|
|
Zárete NAH, Carmo Vieira M, Helmich M, Heid DM, Menegati CT (2010). Produção agroeconômica de três variedades de alface: cultivo com e sem amontoa. Rev. Ciênc. Agron. 41:646-653.
Crossref
|
|