Full Length Research Paper
ABSTRACT
The aim of this work was to study the effect of fungicides and biological agents on the control of white mold (Sclerotinia sclerotiorum) in common beans (cv. Pérola). Nine treatments were applied in six blocks (54 experimental units) using a randomized block design (RBD). The treatments were: T1 (control); T2, Bacillus subtilis strain QST 713 (4 L / ha); T3, B. subtilis strain QST 713 (4L / ha); T4, B. subtilis strain QST 713 (2 L / h); T5, B. subtilis strain QST 713 trifloxystrobin + prothioconazole (4 L / ha, 0.5 L / ha); T6, B. subtilis strain QST 713 trifloxystrobin + prothioconazole (2 L / ha, 0.5 L / ha); T7, B. subtilis strain QST 713 trifloxystrobin + prothioconazole, fluazinam (2 L / ha, 0.5 L / ha, 1 L / ha); T8-trifloxystrobin + prothioconazole, fluazinam (0.5 L / ha, 1L / ha); T9- Trichoderma harzianum, difenoconazole and azoxystrobin fluazinam + (1.5 L / ha, 0.5 L / ha, 1 L / ha). White mold (WM) incidence was evaluated at 39 days after planting (DAP), with subsequent evaluations at 39, 46, 53, 60, 67 and 74 DAP. Average yield from T5, T6, T7 and T8 was statistically higher than in the other treatments and consequently, treatments T7, T8 and T9 had the lowest mean area under disease progress curve values. The combined chemical and biological treatment was an effective white mold management strategy that increased yield and decreased disease incidence in common beans.
Key words: Active ingredient, white mold, Bacillus subtilis, Trichoderma harzianum, triflloxystrobin, prothioconazole.
INTRODUCTION
The common bean [Phaseolus vulgaris L. (Fabaceae)] is one of 55 species in the genus Phaseolus sp. It is one of the most important, oldest and most cultivated crops worldwide. It is extremely important in Brazil where, along with rice, it is a dietary staple (Santos and Gavilanes, 1998).
White mold (WM) in common beans is caused by the soil fungus, Sclerotinia sclerotiorum (Lib.) De Bary (1884) and can trigger epidemics with annual losses exceeding 50%. WM mainly occurs in crops irrigated by central pivot (Oliveira, 2005; Soule et al., 2011). The disease thrives in cool temperatures and / or micro-climatic conditions and during a period of intense bean planting in Brazil called the third growing season (especially in the Southeast and Center-West regions of Brazil). Other conditions that favor the disease include the presence of various fungi hosts, scleroids in the soil and transmission by seeds (Faria et al., 2011).
Innitial symptoms of WM include sparse plants with wilted upper leaves and cottony structures in the stems, leaves and pods formed by fungus mycelium. This last characteristic has led to the name "white-mold" (Paula et al., 2015).
Plant pathogens such as S. sclerotiorum can also colonize seed endosperm. The pathogens can then be transported over long distances by these propagules, proliferate and provide a source of inoculum in new fields. Resistant structures from previous crops or infested soil can also be transported with seeds, machines and agricultural implements, such as tractors, seeders and harvesters when not properly cleaned. Irrigation water, floods and wind can also disseminate the plant pathogen. Infections that begin in the myceliogenic cycle, followed by the ascogenous cycle, can multiply during the crop cycle (secondary cycle) and cause reinfestations, which lead to new resistant structures and increased inocula in the soil (Paula et al., 2015).
The fungus, S. sclerotiorum, identified in 1884, has been studied ever since. It is present throughout the world. It can infect more than 408 species of plants, monocots and dicots (Görgen, 2009). Changes in pigment, leaf wrinkling, wilt, chlorosis, atrophy, necrosis or abscission of parts of the plant are signs of this pathogen-host interaction (Prabhakar et al., 2013).
Solarization is an alternative method for reducing inoculum and controlling fungal plant pathogens in the soil. While this practice has shown promise in small crop areas it may not be practical in larger ones (Ferraz et al., 2003).
Fungicide application is the most common control method because it can be easily adapted to crop management plans and because it effectively prevents, controlls and reduces disease severity (Mueller et al., 2002).
WM can be controlled by physiological resistance and escape mechanisms, a consequence of the growth habit of the plant, which provides favorable soil aeration and climatic conditions. Neither of these mechanisms provide adequate control of the disease (Kim et al., 2000; Kolkman and Kelly, 2002; Huang et al., 2003; Soule et al., 2011). These mechanisms are mainly found in sources of genetic resistance and could be incorporated in commercial cultivars mainly by retro-crossings (Görgen et al., 2003).
Fungicide applications are recommended when flowering begins and again after 10 to 14 days if the disease progresses. Applications via boom sprayers may be hindered by canopy closure between rows and the consequent need for higher volume applications (Tu, 1989). Therefore, to achieve economically viable and effective disease control, growers must pay careful attention to application timing and positioning. The spray should create a uniform layer over the plant surface and act as a barrier to the host-pathogen. Systemic fungicide applications can also provide protection from contact (Oliveira, 2005).
Spraying should be uniformly diffused over the entire plant and soil surface where apotecia develops. Initial spraying should be carried out preventively at the opening of the first flowers. Subsequent applications should occur when apotecia appears and when the crop presents other favorable conditions for disease (Oliveira, 2005). Integrated disease management can lower costs and reduce diverse production risks (Ferreira et al., 2013). Azoxystrobin (estrobirulin chemical group) has been registered for disease control in 32 crops, including beans, and is the active ingredient (ai) in 23 registered commercial products. Another fungicide, trifloxystrobin (strobilulins) has been registered to control diseases in 24 crops, including beans, and is the main in six commercial products; while protioconazol (triazolitione) has been registered to control diseases in 3 crops, in addition to beans, and is the main in two commercial products (Paula et al., 2009).
B. subtilis QST strain 713 is an organic fungicide but is the active ingredient in only one commercial product. While it is not intended for any specific crop it can be used on various (Silva et al., 2015).
Diphenoconazole (triazole group) has been registered in Brazil for WM control in 37 crops, including beans, and 10 commercial products. Diphenoconazole (phenylpyridinylamine group) has been registered for disease control in eight crops, whereas the biological agent Trichoderma harzianum has been registered for disease control in beans and is the main in three commercial products (Agrofit, 2016).
The two main WM control practices in beans involve conventional fungicide use, which is expensive and has strong environmental impacts related to toxic waste (Rocha and Oliveira, 1998). Another practice involves the use of various species of Trichoderma spp. to control not only S. sclerotiorum but various soil pathogens (Lobo and Abreu, 2000).
Our objective is to evaluate the use of chemical and biological managment on Sclerotinia sclerotiorum in irrigated common bean crops (Phaseolus vulgaris).
MATERIALS AND METHODS
We set up our experiment during the dry season of 2015 on an irrigated (central pivot) crop of cv. Pearl at a farm called Fazenda São José in Cristalina, GO, Brazil. The field was situated at 17 ° 5'56 "S and 47 ° 38'44" W (GPS) and at an altitude of 861 m.
The soil was prepared using the no-tillage system and was preceded by soybean and corn crops. The crop was fertilized after planting by broadcast fertilization using 270 kg ha-1 of formulated 05-37-00 potassium chloride (KCl, Triton©). The crop was managed according to Carneiro et al. (2015).
The beans were sown in the first week of October. Nine types of treatments were applied from 1 to 4, times during the crop cycle. Some of these treatments were biological and chemical combinations (Table 1). A randomized block design was used with six replicates, totaling 54 experimental units or plots (Table 2).
Each plot measured 6x6 m (36 m2), spaced at 0.5 m between rows and 0.2 m between plants. The last 0.5 m from the ends of the two central rows was discarded (9 m2 total). The evaluations were carried out on the ten centermost rows (useful area). There were 30 plants plants per row and a total of 300 plants per plot.
White mold incidence (% WM) was evaluated at 39 days after planting (DAP) and again at 46, 53, 60, 67 and 74 DAP (Table 3). The numbers of symptomatic plants (white mold symptoms) were counted five times divided by the total number of evaluated plants (10 plants).
The area under white mold progress curve (AUDPC) was calculated by integrating the disease progress curve for each plot (% white mold incidence at x days), using the formula:
Where, n is the number of the severity ratings, Xi is the severity of the disease and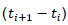 is the number of days between consecutive evaluations (Campbell and Madden, 1990). The value of AUDPC synthesizes all the WM impact assessments into a single value representing the crop-cycle epidemic.
is the number of days between consecutive evaluations (Campbell and Madden, 1990). The value of AUDPC synthesizes all the WM impact assessments into a single value representing the crop-cycle epidemic.
The yield (kg / ha) of the plots was evaluated at 87 DAP (desiccation was carried out 2 days before harvest, affecting 70% of the leaves). The number of plants in 4 rows (2.5 m each) was counted; it was divided by the line spacing used and multiplied by 10, giving the number of plants per ha. Next, the number of pods in 10 consecutive plants in a row was counted and divided by 10, yielding the mean number of pods per plant. Fifty pods were collected, and the number of beans counted. This value was then divided by 50 to find the average number of beans per pod. Next, 1000 beans were weighed. Then, yield was estimated as the mathematical product of the number of plants per ha, the number of pods per plant, the average number of seeds per pod and weight of 1000 beans, divided by 60,000 (Koss and Lewis, 1993).
Control efficiency (CE) is the percent reduction in AUDPC due to a treatment application, relative to the AUDPC values ​​of the control treatment (without applications). Yield efficiency (YE) represents the relationship between yield increases relative to the yield of the control treatments (without application of chemical and biological combinations) (Silva, 2018).
The crop health and yield variables were subjected to analysis of variance and the means compared by the Tukey test at 5% probability (Assistat ® version 7.7 Beta).
RESULTS AND DISCUSSION
No symptoms of white mold were observed during the first evaluation (39 DAP); however, symptoms of fusarium wilt (Fusarium oxysporum f.sp. phaseoli) were observed. Furthermore, mean incidence values did not differ significantly among the various treatments. Similarly, Boechat et al. (2014), using spectral analysis, also did not detect white mold within the same DAP range and suggested that crop phase or the residual effects of previous crop management practices could explain the lack of white mold.
At 46 DAP, when the beans were in the R5 stage, the T5 treatment (B. subtilis strain QST 713 - Serenade© + prothioconazole and trifloxystrobin - Fox© - 4 L ha-1 and 0.5 L ha-1 - V3, V4, R5, R5 +10 days) showed statistically lower incidence of white mold than did the other treatments. Wutzki et al. (2016) found that chemical control applications at these phenological stages did not differ statistically from the control and were therefore not effective. Chromatography–mass spectrometry showed that the bioagent Trichoderma longibrachiatum T6 achieved the same antifungal potential as Trichoderma in the control of Verticillium sp. (Zhang et al., 2018).
At 53 DAP, when the beans were in the R6 stage, the T5, T7, T8 and T9 treatments showed statistically lower incidence of white mold than the other treatments. As expected, the highest incidence occurred in the control (T1) and in the T2, T3 and T4 treatments (Figure 1A, B). Lower performance from the biological treatments is expected, given that this is the first time they had been used during this crop cycle. Continuous use of biological control achieves better results, whereas the first application is only the starting point for results that should continue to improve (Pomella and Ribeiro, 2009).
At 60 DAP, when the beans were in the R6 stage, the T7, T8 and T9 showed the lowest incidence of white mold relative to the other treatments. As expected, the highest incidence of white mold occurred in the control (T1) and in the T2, T3 and T4 treatments (Figure 1C). Meyer et al. (2014) showed that chemical control of WM in soybean crops was efficient and that the active ingredient fluazinam was the most efficient. The chemical treatments in the present study also yielded the best results.
At 67 DAP, when the beans were in the R7 stage, the lowest, statistically different incidence of white mold was found in the T7, T8 and T9 treatments. Again, as expected, the highest incidence occurred in the control (T1) and in T2, T3, T4 and T5 (Figure 1D). Although the T. harzianum treatment showed statistically significant results at this stage we can not say that it was effective, given that it was used in concert with fungicides that were producing much better results. Contrary to Silva et al. (2015), who examined these two biological agents in the control of S. sclerotiorum in lecttuce, we found that T. harzianum provided better control of WM (Silva et al., 2015). Not only was biological control (Trichoderma spp.) of WM studied, but also, edornaviruses, which are specific to fungi, were studied in Vicia faba (Khalifa and Pearson, 2014).
At 74 DAP, when the beans were in the R8 stage, the lowest, statistically different incidence of white mold was found in T8. The highest incidence of white mold occurred in the control (T1) while statistically similar results were found in T2, T3, T4, T5, T6, T8 and T9 (Figure 1E). This shows that biological control of WM was not as effective as chemical control in both seed treatment and in post-emergence applications (Moraes and Teixeira, 2008).
AUDPC, which summarizes the extent of the white mold epidemic, had the lowest value in the T7 treatment, followed in ascending order by T8 and T9, and shortly after by T5 and T6, T2, T3 and T4, which were statistically similar. Finally, the highest incidence of white mold was in the control (T1) (Figure 1F). A commercial product based on Coniothyrium minitans combined with low doses of fungicides was effective at managing white bean mold (Elsheshtawi et al., 2016).
The progress curve expressed the critical limits of disease development in the different treatments, with the control treatment producing the upper limit of incidence (Figure 2). Thus, the best treatment (T7) reduced disease incidence by 0-13 % (control 0-33%) (Figure 2). When pyrisoxazole (rarely used in Brazil) was used to control 166 strains of Sclerotinia sclerotiorum, it provided excellent protection and reduced disease in oleaginous plants (Duan et al., 2018). The fungicides procymidone and fluazinam (commonly used in Brazil) combined with benzalkonium chloride were more efficient in controlling WM in soybeans (73.1 - 71.6%, 2010 crop; 75.7 - 77.6 %, 2011 crop) than isolated applications of T. harzianum (Sumida et al., 2015).
From 48 to 53 DAP, disease development was considered critical due to progressive growth in all the chemical treatments. In the T8 treatment, reductions in incidence began to decrease at 60 DAP (Figure 2). Single chemical applications are not effective over long crop periods; however, efficacy can be extended by combining treatments chemical and biological (Moraes et al., 2008; Mueller et al., 2002; Paula Junior et al., 2006).
After harvesting, we compared bean yields from the various treatments. The statistically highest yields were in T8 (3590 kg / ha) and T5, T6, T7 and T9, which were statistically similar. The control (T1) and T2, T3 and T4 produced the lowest yields (<3490 kg / ha) (Figure 3). Chemical fungicides provided better WM control and consequently higher yields. Similar conclusions were drawn by Paula Junior et al. (2009).
The highest control efficiency, as measured by AUDPC, was observed in T7 (77.9%), followed by T8 (72.4%) and then T9 (67.8 %), which demonstrates that control efficiency greater than 50% was achieved in these treatments (Carneiro et al., 2015).
The highest yield efficiency was T8 (51.8%), followed by T7 (35.7 %), T5 (32.8 %), T6 (30.2 %), T9 (28.2 %), T3 (14.6 %), T2 (13.2 %) and T4 with the lowest percentage (7.2 %) (Table 1), showing that control efficiency is linked to yield (that is the lower the WM intensity, the higher the yield). The fact that this was the first time these biological controls were used to control this pathogen may partly explain why these treatments produced the lowest yield and control efficiencies (Vinale et al., 2008).
The highest average yield was 3178 kg ha-1 or 53 sc ha-1 for treatment T8 (trifloxystrobin + prothioconazole - Fox© and fluazinam - Frowncide©) (Table 1). The treatment with the combined chemical-biological application yielded 1085 kg ha-1 (18 sc ha-1) more than the untreated control. Thus, 100 ha, under the same conditions, could yield an additional 108,500 kg (1800 sc 100 ha-1) / 100 ha of beans, which, at current prices (R$ 205.00 sc) would provide an additional R$ 369,000.00. Given spraying costs per hectare of R$ 11.10 (Richetti and Roese, 2008), the spraying costs on 100 hectares would be R $ 1110.00 per application.
In the T8 treatment (Table 1), the fungicide trifloxystrobin + prothioconazole - Fox© for 1 h costs R$ 65.00 per hectare (R$ 130.00 per liter; dosage 0.5 L / ha-1) or R$ 6,500.00 per application on 100 ha. Similarly, the fungicide fluazinam - Frowncide© also costs R$ 65.00 per ha (R$ 130.00 per liter; dosage of 0.5 L / ha), which would cost an additional R$ 6500.00 per application on 100 ha. Thus, a single application on 100 ha of the fungicides in the T8 treatment would cost R$ 13,000.00 (Carneiro et al., 2015).
Finally, the total cost of three applications of T8 on 100 ha would be R$ 58,500.00 (3 x (spraying cost of R$ 1110 + fungicide cost of R$ 13,000). Therefore, the net revenue on 100 ha gained by using the T8 treatment, rather than the untreated control no treatment, would be R$ 307,170.00 per 100 ha (Carneiro et al., 2015).
Yield increases in the experiment were explained by AUDPC (84.3%) (Figure 4), including highly correlated variables (growth rate of -0.6143% day-1) fit to a linear model. The control treatment (without any applications) showed that higher AUDPC was related to lower yields.
Contrary to our study, isolated fungicide active ingredients (not mixed with other active ingredients) and isolated treatments of T. harzianum in two consecutive harvests were shown to be more efficient at controlling the severity and incidence of WM and improving yield than treatments containing pure fungicides (fluazinam) in the 2009-2010 crop (Sumida et al., 2015).
T7 and T9 were strongly correlated with higher yield and lower AUDPC (Figure 4). Decreased WM, which was influenced by physiological resistance and plant architecture, had little influence on yield but reduced AUDPC (Görgen et al., 2003).
CONCLUSION
Single applications of biological control agents (T2, T3 and T4), applied at different rates and times, had statistically similar effects on WM incidence in common beans throughout the evaluation period.
A combination of a biological control agent (B. subtilis) and two active chemical control ingredients (trifloxystrobin + prothioconazole and fluazinam) produced the greatest reduction in AUDPC. The treatments using only biological control agents produced greater reductions in AUDPC than did the treatment without a combination of controls strategies.
T8 (three applications), with two chemical treatments and 3 applications over the bean growth cycle, produced numerically higher yields that were statistically equal to the yields of the treatments with combinations of biological and chemical agents (T5, T6, T7 and T9, four applications).
The highest control efficiency related AUDPC (77.9%) and yield efficiency (51.8%) were in T7 and T8 respectively.
The yield increases from the combined chemical and biological treatments reduced AUDPC by 84.3%. T8 increased yields, lowered final production costs and reduced the incidence of white mold, which the crop converted into yield gains.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors thank the Instituto Federal Goiano campus Urutaí and Consulting Firm RC Consulting represented by Sara Aparecida Cunha Teixeira and Roberto Vitor Inácio
REFERENCES
|
Agrofit (2016). Agrofit Disponível in |
|
|
Boechat T, Carvalho S, Paula J, Queiroz M, Teixeira H (2014). Detecção do mofo-branco no feijoeiro, utilizando características espectrais. Revista Ceres. Viçosa 61(6):907-915. |
|
|
Campbell L, Madden V (1990). Introduction to Plant Disease Epidemiology. Wiley-Interscience, NY. P. 532. |
|
|
Carneiro JE, Paula Junior TJ, Borém A (2015). Feijão do plantio a colheita. Universidade Federal de Viçosa, Editora UFV, Viçosa, MG, 384 p. |
|
|
Duan Y, Li T, Xiao X, Wu J, Li S, Wang J, Zhou M (2018). Pharmacological characteristics of the novel fungicide pyrisoxazole against Sclerotinia sclerotiorum. Pesticide Biochemistry and Physiology 147:45-50. |
|
|
Faria C, Coelho C, Pereira E, Cintra G, Araújo S (2011). Transformação de feijoeiro com o gene oxalato oxidase para resistência ao mofo branco causado por Sclerotinia sclerotiorum (Lib.) de Bary. Congresso Nacional de Pesquisa de Feijão P 10. |
|
|
Ferraz LCL, Bergamin Filho A, Amorim L, Nasser LCB (2003). Viabilidade de Sclerotinia sclerotiorum após a solarização do solo na presença de cobertura morta. Fitopatologia Brasileira 28:17-26. |
|
|
Görgen CA, Civardi EA, Lobo Junior M, Carneiro LC, Oliveira LA, Huang HC, Mundel HH, Ericson RS (2003). Effect of physiological resistance and plant architecture on yield of dry bean under disease pressure of white mold (Sclerotinia sclerotiorum). Plant Protection Bulletin 45:169-176. |
|
|
Khalifa ME, Pearson MN (2014). Molecular characterisation of an edornavirus infecting the phytopathogen Sclerotinia sclerotiorum. Virus Research 189(2):303-309. |
|
|
Kim HS, Sneller CH, Diers BW (2000). Inheritance of partial resistance to Sclerotinia stem rot in soybean. Crop Science 40:55-61. |
|
|
Kolkman JM, Kelly JD (2002). Agronomic traits affecting resistance to white mold in common bean. Crop Science 42:693-699. |
|
|
Koss JE, Lewis DA (1993). Productivity or efficiency measuring what we really want. National Productivity Review 12:273-295. |
|
|
Meyer MC, Campos HD, Henning AA, Utiamada CM, Pimenta CB, Godoy CV, Jaccoud Filho DS, Miguel-Wruck DS, Ramos Júnior E, Borges EP, Lopes ION, Nunes Junior J, Silva LHCP, Ito MA, Martins MC, Andrade PJ, Lopes PVL, Zito RK, Furlan SH, Venancio SW (2014). Eficiência de fungicidas para controle de mofo branco (Sclerotinia sclerotiorum) em soja, na safra 2008/2009 – resultados sumarizados e individuais dos ensaios cooperativos. In: Meyer MC, Campos HD, Godoy CV, Utiamada CM, Ed(s). Ensaios cooperativos de controle químico de mofo branco na cultura da soja: safras 2009 a 2012. Embrapa soja, Londrina, doc. 345:17-30. |
|
|
Moraes ER, Teixeira IR, Souza DLM (2008). Associação fungicidas-agente biológico (Trichoderma sp.) no controle do mofo branco do feijoeiro. Anais do Congresso nacional de Feijão, Ed.8, Campinas, n. 236-052. |
|
|
Mueller DS, Dorrance AE, Derksen RC, Ozkan E, Kurle JE, Grau CR, Gaska JM, Hartman GL, Bradley CA, Pedersen WL (2002) Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Disease 86:26-31. |
|
|
Oliveira SHF (2005). Manejo do mofo-branco. DBO Agrotecnologia 2:8-13. |
|
|
Paula Júnior TJ, Vieira RF, Lobo Júnior M, Morandi MAB, Carneiro JES, Zambolim L (2006). Manejo integrado do mofo-branco do feijoeiro. Viçosa – MG: Epamig, P 48. |
|
|
Paula Júnior TJ, Vieira RF, Rocha PRR, Bernardes A, Costa EL, Carneiro JES, Vale FXR, Zambolim L (2009). White mold intensity on common bean in response to plant density, irrigation frequency, grass mulching, Trichoderma spp. and fungicide. Summa Phytopathologica 35:44-48. |
|
|
Paula Junior TJ, Vieira RS, Teixeira H, Lobo Junior M, Wendland A (2015). Doenças do feijoeiro, estratégias integradas de manejo. In: Carneiro, J.E., Paula Júnior, T.J., Borém, A. Feijão do Plantio a Colheita. UFV: Viçosa, P 384. |
|
|
Pomella AWV, Ribeiro RTS (2009). Controle biológico com Trichoderma em grandes culturas - uma visão empresarial. In: Bettiol W, Morandi MAB, Ed(s). Biocontrole de doenças de plantas: uso e perspectivas. Embrapa Meio Ambiente 1:239-244. |
|
|
Prabhakar M, Prasad YG, Desai S, Thirupathi M, Gopika K, Rao GR, Venkateswarlu B (2013). Hyperspectral remote sensing of yellow mosaic severity and associated pigment losses in Vigna mungo using multinomial logistic regression models. Crop Protection 45:132-140. |
|
|
Richetti A, Roese AD (2008). Custo do controle químico da ferrugem asiática da soja em Dourados, MS, para a safra 2008/09. Embrapa, Dourados, MS, Comunicado Técnico n° 150. |
|
|
Santos JB, Gavilanes ML (1998). Botânica. In: Borém, A., Paula Júnior, T.J., Vieira C. (eds) Feijão: aspectos gerais e cultura no estado de Minas. UFV, Viçosa, pp. 55-79. |
|
|
Silva GBP, Heckler LI, Santos RF, Durigon MR, Blume E (2015). Identificação e utilização de Trichoderma spp. Armazenados e nativos no biocontrole de sclerotinia sclerotiorum. Revista Caatinga 28(4):33-42. |
|
|
Soule M, Porter L, Medina J, Santana GP, Blair MW, Miklas PN (2011). Comparative QTL map for white mold resistance in common bean, and characterization of partial resistance in dry bean lines VA19 and I9365-31. Crop Science 51:12-139. |
|
|
Sumida CH, Canteri MG, Peitl DC, Tibolla F, Orsini IP, Araújo FA, Chagas DF, Calvos NS (2015). Chemical and biological control os sclerotinia stem rot in the coybena crop. Ciência Rural 45(5):760-766. |
|
|
Tu JC (1989). Management of white mold of white beans in Ontário. Plant Disease 73:281-285. |
|
|
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Wooa SL, Lorito M (2008). Trichoderma plant pathogen interactions. Soil Biology and Biochemistry 40:1-10. |
|
|
Wutzki CR, Jaccoud Filho DS, Berger Neto A, Tullio HE, Juliatti FC, Nascimento AJ (2016). Reduction of white mold level on soybean by fungicide management strategies. Bioscience Journal 32(3):642-651. |
|
|
Zhang S, Xu B, Zhang J, Gan Y (2018). Identification of the antifungal activity of Trichoderma longibrachiatum T6 and assessment of bioactive substances in controlling phytopathgens. Pesticide Biochemistry and Physiology 147(1):59-66. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0