ABSTRACT
Phytosociological surveys are basis for weed management in agricultural crops. We aimed in this study to survey weed within a cassava cultivation field in the city of Vitória da Conquista, Southwestern Bahia, Brazil. The crop was grown for 18 months (from January 2013 to July 2014), with samples at 35, 70 and 105 days after planting (first year cultivation), and at 350, 385 and 420 days after planting (second year cultivation). Sampling was performed according to inventory square method, in which a 0.25 m2 iron frame is thrown randomly on the cropland. Then, weed within this metal square area are cut at ground level, identified, quantified and afterwards placed into an oven at 65°C during 72 h to obtain dry mass of each species. Evaluated phytosociological parameters were frequency, relative frequency, density, relative density, abundance, relative abundance and importance value index. The main identified families in the survey were Malvaceae, Asteraceae and Poaceae. In total, it was assessed 14 families, 32 genera and 38 species of weeds. The highest importance value index was found for Sida rhombifolia, Cynodon dactylon and Brachiaria plantaginea. Regarding dry mass, Panicum maximum, B. plantaginea and S. rhombifolia had the largest values. It was concluded that weed control methods must focus on species and consider reinfestations in the second crop year.
Key words: Manihot esculenta Crantz, infesting community, competition, weed-competition.
Cassava (Manihot esculenta Crantz) is a crop with great social and economic importance in Brazil, being cultivated in more than 1.9 million hectares and its production is intended mainly to the production of flour, starch and in natura consumption (IBGE, 2013). The great social importance of this crop is due to its exploitation in regions with dry seasons and/or poor soils, where occur the lowest levels of human development index (HDI) of Brazil and the world (Silva et al., 2014).
On national scenario, Bahia is one of the main producing states, generating near 1.85 million tons, 8.72% of national production, which is 21.22 million tons, and a yield of 13.91 tons ha-1 (IBGE, 2014). Vitória da Conquista city is home to a prominent micro region of cassava production, accounting for approximately 10% of the state production (IBGE, 2008). However, despite its importance, the root yield is considered low when compared with the crop production potential of up to about 90 t ha-1 of roots (Cock et al., 1979).
Low production rates are common in other Brazilian regions, in which the main limiting factors are; little adoption of adequate agronomic technology, low fertility of soils, low quality planting material, unproductive varieties and/or poorly adapted to the region, weed competition, among others (Cardoso et al., 2013).
Weed in cassava cultivation has been reported as one of the main factors affecting crop yield. According to Albuquerque et al. (2008), root yield can be reduced by more than 90% in absence of weed control. This is due mainly to a slow initial growth of cassava plants, which facilitates weed species development, favoring the competition for water, light, nutrients, carbon dioxide and physical space (Azevêdo et al., 2000). In addition, cassava harvest can occur up to two years after planting, when roots are delivered to processing industry (Silva et al., 2012). Because of long cultivation and the soil partial covering by the plant, several weed infestations can occur within the planting area, which might increase crop yield losses (Johanns and Contiero, 2006). According to Cruz and Pelacani (1993), shading by weeds increases plant height, without increase in shoot biomass accumulation and reduction of leaf area index. They concluded that, with less exposure to light, cassava stem and leaf dry mass and root yield are compromised.
For a proper weed management, local species should be identified, as well as the knowledge of those which are most important (Oliveira and Freitas, 2008). Such information can be achieved by means of phytosociological surveys (Tuffi Santos et al., 2004). From this survey, it is possible to assess the plant composition and their frequency, density, abundance and relative importance index of the species and determine an optimal period for controlling them; which can increase control efficiency, streamline costs and reduce environmental impact of cassava production (Isaac and Guimarães, 2008; Guglieri et al., 2009).
Given the above, the authors aimed to identify and quantify the main species of weed in cassava cultivation field in Vitória da Conquista, Bahia, Brazil.
The survey was carried out from February 2013 to July 2014, in the experimental field of the State University of the Southwestern Bahia, Campus in Vitória da Conquista, BA, Brazil. It located at 14° 51’ S, 40° 50’ W geographical coordinates and at 941-m average altitude. According Köppen, local climate is classified as Cwa type (high-altitude tropical climate) with annual rainfall of 741 mm. Local soil is classified as Dystrophic Yellow Oxisol, with loam-clay-sand texture over a plain relief (EMBRAPA, 2006).
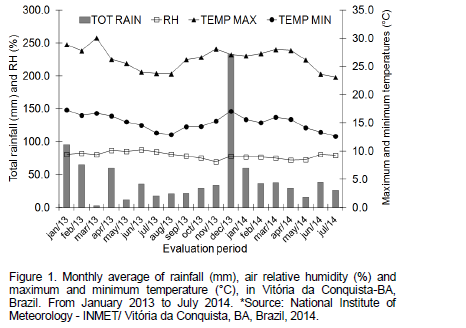
Figure 1 displays weather data of the assessed period related to rainfall, relative humidity, maximum and minimum temperature. Soil preparation was made in conventional way with plowing, harrowings and furrowing. Fertilization was performed according soil analysis and recommendations for cassava crop (Nogueira and Gomes, 1999), applying in the first year 40 kg ha-1 P2O5, directly in planting furrow; 70 kg ha-1 N and 30 kg ha-1 K2O, in top-dressing, 60 days after planting. In the second year, 60 Kg N and 60 Kg K2O were applied as top-dressing in the beginning of the rainy season.
Planting was manually performed in January 2013, using ‘Caitité’ variety with about 2 to 3 cm stem diameter, 20 cm length and seven buds. Plant spacing was 1.0 m between rows and 0.6 m between plants, totaling 16,666 plants ha-1.
Crop was grown for 18 months with evaluations every 35 days since planting; thus, there were evaluations at 35, 70 and 105 days after planting. Weeds were characterized at the crop initial stage (first year), and at 350, 385 and 420 days, in the second crop year, when weed reinfestation might occur due to when rainy season begin.
Weeds were identified and quantified by the inventory square method (Braun-Blanquet, 1979). A metal squared frame of 0.5 × 0.5 m (0.25 m2) was thrown randomly within plots. Each plot had 33.6 m2 (8.4 m long × 4 m wide), totaling 604.8 m2. Eighteen samplings were performed for each period, with 108 samplings.
Weed from each sample were removed by cutting shoot at the ground level, packaging them in paper bags and transferred to the Laboratory of Biotechnology, where they were identified based on specialized bibliography (Lorenzi, 2008; Kissmann and Groth, 2000). Then, they were counted and dried in an air-forced oven at 65°C for 72 h for dry matter measurements.
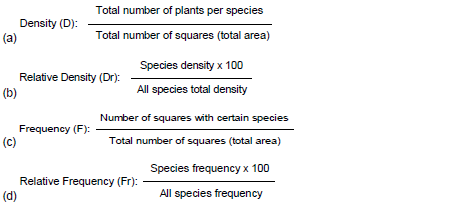
From species identification, phytosociological parameters were determined according to Curtis and Mcintosh (1950) and Mueller-Dombois and Ellenberg (1974). Such parameters were:
(g) Importance Value Index (IVI): Relative frequency + relative density + relative abundance
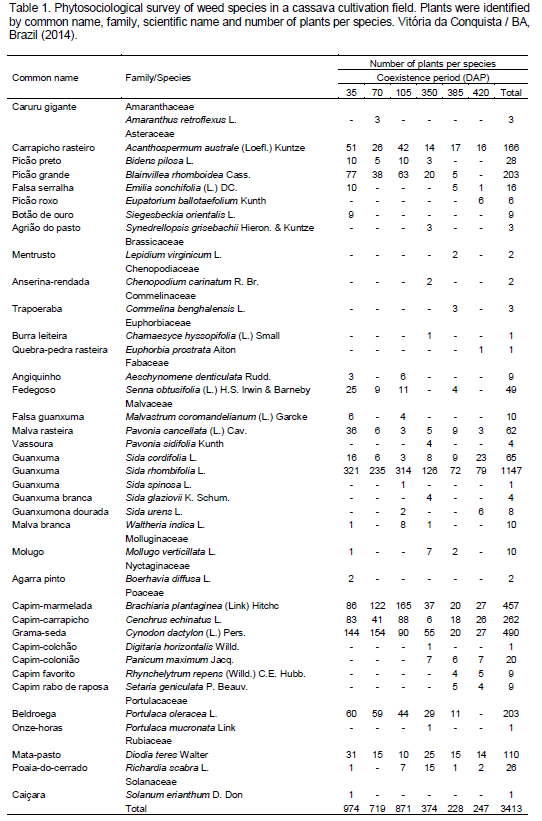
According to the phytosociological survey, local weed community was composed by 38 species, divided into 32 genera and 14 families in a total of 3413 plants. Concerning species number, Malvaceae (nine), Asteraceae and Poaceae (both seven) can be highlighted, which had 60.5% of total number of weed species (Table 1). Some families found in this survey are common in cassava crops, being also reported in other surveys such as Otsubo et al. (2002), Albuquerque et al. (2008) and Guglieri et al. (2009), point out these families as the richest families in weed species found in cassava cultivations.
Weed community was considered heterogeneous, when compared to Albuquerque et al. (2014) research, who evaluated weed occurrence in cassava fields of Roraima (Boa Vista/ RR, Brazil). The authors found 27 weed species distributed into 21 genera and 8 families. Huziwara et al. (2009) surveyed weed communities in Campos de Goytacazes-RJ and identified only 10 species from nine genera and nine families in cassava crops.
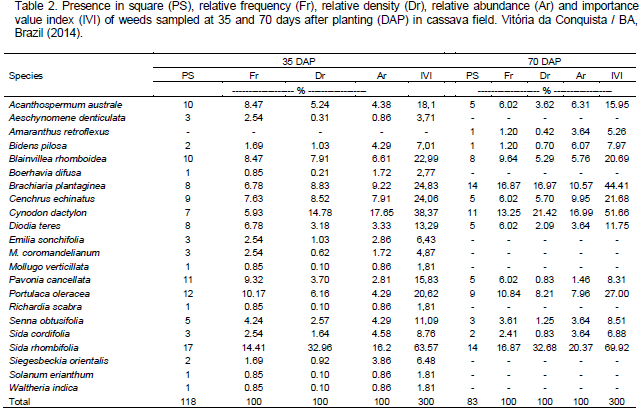
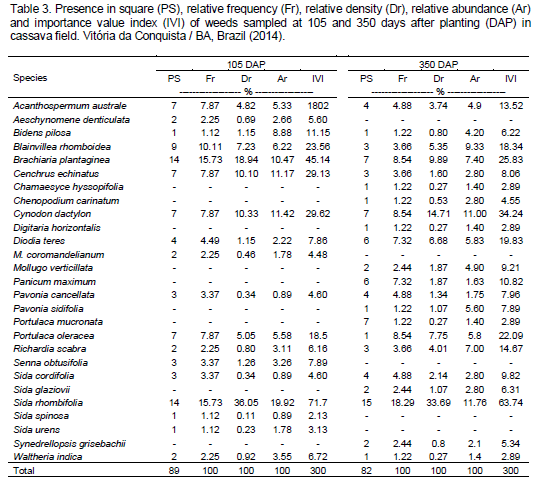
S. rhombifolia, Cynodon dactylon and Brachiaria plantaginea were predominant with a high number of plants along the six evaluations. S. rhombifolia was 33.60% of all surveyed plants (1.147), followed by C. dactylon with 14.36% (490) and B. plantaginea with 13.39% (457) (Table 1).
Concerning the phytosociological parameters (Tables 2, 3 and 4), it can be seen that S. rhombifolia had relative abundance value smaller than C. dactylon only at 35 DAP, presenting the highest phytosociological index of the survey, and IVI values varying from 58.92 to 71.7% and average of 65.03%.
S. rhombifolia widespread occurrence within experimental area can be attributed to its high infestation potential, once the species has high seed production and ease to disperse. As stated by Pitelli (1985), relative importance degree of infesting species at a certain location is given by balance of phytosociological indexes; therefore, it is the most weighted evaluation of a plant population. Furthermore, unsuitable plant density, slow growth varieties and inadequate planting area management are related to increased competition between weeds and cassava plants (Almendra, 2005).
S. rhombifolia grows together with annual and perennial crops, being extremely competitive due to its extensive root system that may reach 50 cm depth (Lorenzi, 2008; Kissmann and Groth, 2000). Some studies reported that this plant might produce near 28.2 thousand seeds m-2 during a single summer cycle as soybean crop weed (Fleck et al., 2003). The species was mentioned as weed in cassava plantations by Azevêdo et al. (2000) and Albuquerque et al. (2008), in corn field (Macedo et al., 2003), sugarcane (Oliveira and Freitas, 2008) and soybean (Voll et al., 2005).
C. dactylon and B. plantaginea alternated for the highest phytosociological indexes, and B. plantaginea showed the highest relative frequency, while C. dactylon, the highest relative density and abundance. These indexes reflect that in cassava field, C. dactylon keeps concentrated in “spots”. This weed is considered one of the most important grassy weeds, mainly for sugarcane crop in Brazil as it is hard to eradicate them once established (Carbonari et al., 2005; Ferreira et al., 2011). This species presented high value among the evaluated parameters, which may be due to its underground breeding structures, which enables growth retaken after some days. Moreover, Cardoso et al. (2013), who performed a phytosociological survey in the studied city, mentioned it as one of the main infesting plants in cassava cultivation.
B. plantaginea demonstrated great adaptability and aggressiveness and had high phytosociological indexes in all evaluations. This African grass is mainly propagated through seeds with primary dormancy during maturation (Lorenzi, 2008). This way germination is spread all over the time being of difficult control (Kissmann, 1997).
Compared to the first crop year, the second showed a reduction in weed number, frequency, density and abundance (Tables 2, 3 and 4). This reduction showed greater competition during the initial phase of cassava development. This fact underscores the importance of maintaining the crop free of weeds during this period. This fact is possibly connected to a slow initial growth of cassava plants, which associated with a wide planting space, provides low competitive ability with weeds, especially with regard to soil coverage, allowing weeds to emerge along a period of time (Lorenzi and Dias, 1993). Biffe et al. (2010) obtained similar results, in which they found that weed interference in cassava is greater between 18 and 100 days after planting.
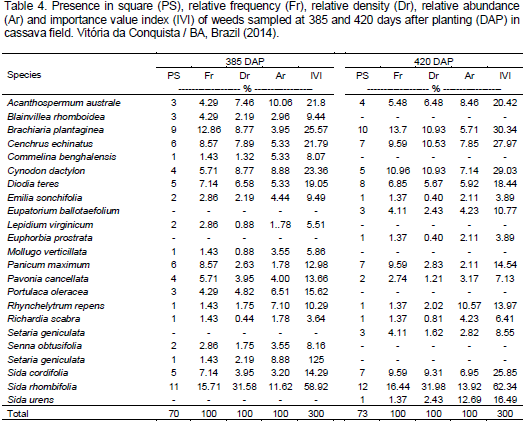
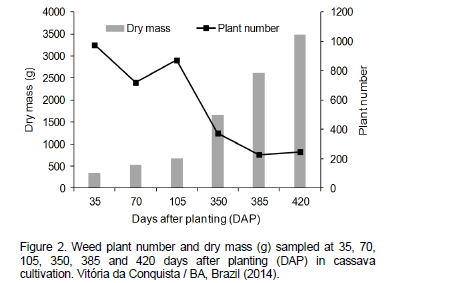
Despite the reduction in plant number in the second year, there was a steady increase in dry mass of the remaining weeds along the evaluations (Figure 2). This behavior can be explained by cassava shading on weeds or competition among weeds. According to Radosevich et al. (1996), as weed density and development increases, especially those that germinated and emerged at the beginning of the crop cycle such as cassava, intraspecific and interspecific competition increases, so that the highest and most developed weeds become dominant, while the smaller ones are removed or die. This behavior explains plant number reduction and weed dry mass increase in the second year.
Table 5 shows averages of dry mass by weed species, in which the greatest values belong to Panicum maximum, B. plantaginea and S. rhombifolia, totaling 829.9, 437.2 and 278.5 g m-2, respectively.
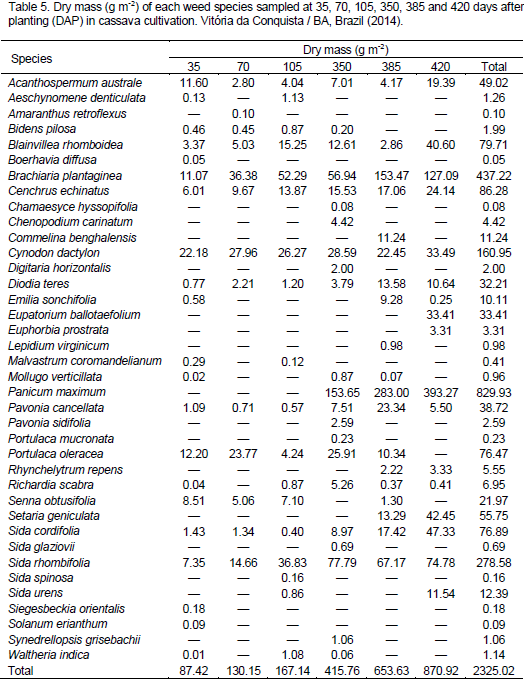
Among the grasses with higher dry matter accumulation, P. maximum had significant growth in second crop year (350, 385 and 420 DAP), showing its great competitive power as a function of biomass production capacity compared to the other species. Such an occurrence is probably related to the weed presence in neighboring areas, defoliation of cassava plants during maturation and seed dispersal of this species, applied fertilizer and the beginning of rainy season. These conditions certainly favored its establishment and development within the area, once the weed is very demanding in light, fertility and soil moisture. On the other hand, B. plantaginea had high dry matter accumulation in all evaluations, which indicates its good adaptation to the environment. According to Maciel et al. (2010), several species of Poaceae family are perennial and produce many seeds, increasing its spread and colonization potential at different environments. S. rhombifolia and C. dactylon showed dry matter values lower than the first two and had the highest phytosociological indices (Table 5).
Several other authors conducted phytosociological surveys in the cassava crops along Brazil. In these surveys, they identified numerous weed species of distinct genera and families (Azevêdo et al., 2000; Johanns and Contiero, 2006; Albuquerque et al., 2008; Guglieri et al., 2009; Huziwara et al., 2009; Pinotti et al., 2010; Biffe et al., 2010; Cardoso et al., 2013; Albuquerque et al., 2014). The species identified in the study cited above varied according to planting period, management, location and land history. Although, there are common species in various parts of the country, each site had a peculiarity regarding the dominant species. In the present study, we verified few predominant species (S. rhombifolia, C. dactylon and B. plantaginea). This fact can be attributed to rainfall irregularities, high temperatures and soil type. Therefore, such studies should be performed in several producing regions, since weed community composition differs among seasons and different places.
Significant part of cassava production costs can be attributed to weed control, which may vary according to weed species and population densities. In this context, the knowledge of weed community distribution and composition is important for solving problems related to potential infestations, being directly connected to the control strategy (Pinotti et al., 2010; Aguiar et al., 2011). Thus, understanding the weed population dynamics based on phytosociological parameters is essential for an ideal crop management (Oliveira and Freitas, 2008).
Despite the greater number of plants was observed for up to 105 days after planting cassava (75.12% of total), grasses in the second year were significant and had high dry matter values. Therefore, in crops with aggressive grass species such as P. maximum and B. plantaginea, as found in this survey, we recommend a weed management plan taking into account the two crop years. Given the above mentioned, it can be said that dry matter data complemented the phytosociological survey.
The weed community composition was considered heterogeneous presenting 38 species belonging to 32 genera and 14 families. The families with the largest number of species identified were Malvaceae, Asteraceae and Poaceae, predominating the species S. rhombifolia, C. dactylon and B. plantaginea.
The occurrence of grasses such as P. maximum and B. plantaginea during the crop cycle indicates the need for a weed management plan focusing on both crop years.
The authors did not pronounce any conflict of interest.
The authors thank the Postgraduate Program of Agricultural Sciences of the Universidade Estadual do Sudoeste da Bahia (State University of Southwestern Bahia), the staff of the Laboratory of Biotechnology and the Departamento de Campo Agropecuário – DICAP (Department of Agricultural Field).
REFERENCES
Aguiar EB, Bicudo SJ, Curcelli F, Figueiredo PG, Cruz SCS (2011). Épocas de poda e produtividade da mandioca. Pesquisa Agropec. Bras. 46(11):1463-1470.
Crossref |
|
|
Albuquerque JAA, Evangelista MO, Mates APK, Alves JMA, Oliveira NT, Sediyama T, Silva AA (2014). Occurrence of weeds in cassava savanna plantations in Roraima. Planta Daninha 32(1):91-98.
Crossref |
|
|
Albuquerque JAA, Sediyama T, Silva AA, Carneiro JES, Cecon PR, Alves JMA (2008). Interferência de plantas daninhas sobre a produtividade da mandioca (Manihot esculenta). Planta Daninha 26(2):279-289.
Crossref |
|
|
|
Almendra AA (2005). Avaliação de três cultivares de mandioca de mesa (Manihot esculenta Crantz) submetidas ao controle de plantas daninhas. 2005. 29f. Dissertação (Mestrado em Agronomia), Universidade Federal do Piauí, Teresina, Piauí. |
|
|
|
Azevêdo CLL, Carvalho JEB, Lopes LC, Araújo AMA (2000). Levantamento de plantas daninhas na cultura da mandioca, em um ecossistema semi-árido do Estado da Bahia. Magistra 12(1):41-49. |
|
|
Biffe DF, Constantin J, Oliveira Júnior RS, Franchini LHN, Rios FA, Blainski E, Arantes JGZ, Alonso DG, Cavalieri SD (2010). Período de interferência de plantas daninhas em mandioca (Manihot esculenta) no Noroeste do Paraná. Planta Daninha 28(3):471-478.
Crossref |
|
|
|
Braun-Blanquet V (1979). Fitosociología: bases para el estudio de las comunidades vegetales. Madrid: H. Blume P. 820. |
|
|
Carbonari CA, Martins D, Marchi SR, Cardoso LR (2005). Efeito de surfatantes e pontas de pulverização na deposição de calda de pulverização em plantas de grama-seda. Planta Daninha 23(4):725-729.
Crossref |
|
|
|
Cardoso AD, Viana AES, Barbosa RP, Teixeira PRG, Cardoso Júnior NS, Fogaça JJNL (2013). Levantamento fitossociológico de plantas daninhas na cultura da mandioca em Vitória da Conquista, Bahia. Biosci. J. 29(5):1130-1140. |
|
|
Cock JH, Franklin D, Sandoval G, Juri P (1979). The ideal cassava plant for maximum yield. Crop Sci. 19:271-279.
Crossref |
|
|
Curtis JT, Mcintosh RP (1950). The interrelations of certain analytic and synthetic Phytosociological characters. Ecol. 31:434-455.
Crossref |
|
|
|
EMBRAPA EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA – EMBRAPA (2006). Sistema brasileiro de classificação de solos. Brasília: Embrapa Produção de Informação; Rio de Janeiro: Embrapa Solos P. 412. |
|
|
Ferreira RV, Contato ED, Kuva MA, Ferraudo AS, Alves PLCA, Magario FB, Salgado TP (2011). Organização das comunidades infestantes de plantas daninhas na cultura da cana-de-açúcar em agrupamentos-padrão. Planta Daninha 29(2):363-371.
Crossref |
|
|
Fleck NG, Rizzardi MA, Agostinetto D, Vidal RA (2003). Produção de sementes por picão-preto e guanxuma em função de densidades das plantas daninhas e da época de semeadura da soja. Planta Daninha 21(2):191-202.
Crossref |
|
|
|
Guglieri A, Caporal FJM, Vinci-Carlos HC, Pinto BEM (2009). Fitossociologia de plantas espontâneas em um mandiocal implantado em pastagem cultivada em Mato Grosso do Sul, Brasil. Rev. Ciênc. Agrária 51:127-141. |
|
|
|
Huziwara E, Ogliari J, Freitas SP, Paes HMF, Lemos GCS (2009). Levantamento fitossociológico de plantas daninhas na cultura da mandioca no município de Campos dos Goytacazes, RJ. Rev. Raízes Amidos Trop. 5(1):468-472.
View
|
|
|
|
INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA – (IBGE) (2014). Disponível em: |
|
|
|
INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA – (IBGE) (2013). Disponível em: |
|
|
|
INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA – (IBGE) (2008). Disponível em: |
|
|
|
INSTITUTO NACIONAL DE METEOROLOGIA – (INMET) / Vitória da Conquista, BA, 2014. |
|
|
Isaac RA, Guimarães SC (2008). Banco de sementes e flora emergente de plantas daninhas. Planta Daninha 26(3):521-530.
Crossref |
|
|
|
Johanns O, Contiero R (2006). Efeitos de diferentes períodos de controle e convivência de plantas daninhas com a cultura da mandioca. Rev. Ciênc. Agronôm. 37(3):326-331.
View
|
|
|
|
Kissmann KG (1997). Plantas infestantes e nocivas. 2.ed. São Paulo: Basf Brasileira pp. 415-420. |
|
|
|
Kissmann KG, Groth D (2000). Plantas infestantes e nocivas. 2.ed. São Paulo: BASF, Tomo III, P. 723. |
|
|
|
Lorenzi H (2008). Plantas daninhas do Brasil: terrestres, aquáticas, parasitas e tóxicas. 4.ed. Nova Odessa: Plantarum P. 640. |
|
|
Lorenzi JO, Dias CAC (1993). Cultura da mandioca. Campinas: SAA/CATI, (Boletim técnico, 211). P. 41.
PMCid:PMC47625 |
|
|
Macedo JF, Brandão M, Lara JFR (2003). Plantas daninhas na pós-colheita de milho nas várzeas do Rio São Francisco, em Minas Gerais. Planta Daninha. 21(2):239-248.
Crossref |
|
|
Maciel CDC, Poletine JP, Oliveira Neto AM, Guerra N, Justiniano W (2010). Levantamento fitossociológico de plantas daninhas em calçadas do município de Paraguaçu Paulista-SP. Planta Daninha 28(1):53-60.
Crossref |
|
|
|
Mueller-Dombois D, Ellenberg H (1974). Aims and methods of vegetation ecology. New York: John Wiley e Sons. P. 547. |
|
|
|
Nogueira FD, Gomes J, Mandioca de C (1999). In Ribeiro AC et al. (Ed.). Recomendações para uso de corretivos e fertilizantes em Minas Gerais. 5ª Aproximação. pp. 312-313. |
|
|
Oliveira AR, Freitas SP (2008). Levantamento fitossociológico de plantas daninhas em áreas de produção de cana-de-açúcar. Planta Daninha 26(1):33-46.
Crossref |
|
|
|
Otsubo AA, Mercante FM, Martins CS (Ed.) (2002). Aspectos do cultivo da mandioca em mato Grosso do Sul. Dourados: Embrapa Agropecuária Oeste; Campo grande: UNIDERP. P. 219. |
|
|
|
Pinotti EB, Bicudo SJ, Curcelli F, Dourado WS (2010). Levantamento florístico de plantas daninhas na cultura da mandioca no município de Pompéia – SP. Revista Raízes e Amidos Tropicais. 6:120-125. |
|
|
|
Pitelli RA (1985). Interferência de plantas daninhas em cultivos agrícolas. Informe Agropecuário. 11(1):16-26. |
|
|
|
Radosevich SR, Holt J, Ghersa C (1996). Physiological aspects of competition. In: Radosevich SR, Holt J, Ghersa C. (Eds.) Weed ecology: implications for managements. New York: John Willey & Sons, pp. 217-301. |
|
|
Silva DV, Santos JB, Ferreira EA, Silva AA, França AC, Sediyama T (2012). Manejo de plantas daninhas na cultura da mandioca. Planta Daninha 30(4):901-910.
Crossref |
|
|
Silva DV, Silveira HM, Ferreira EA, Carvalho FP, Castro Neto MD, Silva AA, Sediyama T (2014). Aspectos fisiológicos da mandioca após a aplicação dos herbicidas fluazifop-p-butil e fomesafen. Rev. Ceres 61(2):178-183.
Crossref |
|
|
Tuffi Santos LD, Santos IC, Oliveira CH, Santos MV, Ferreira FA, Queiroz DS (2004). Levantamento fitossociológico em pastagens degradadas sob condições de várzea. Planta Daninha 22(3):343-349.
Crossref |
|
|
|
Voll E, Gazziero DLP, Brighenti AM, Adegas FS, Gaudêncio CA, Voll CE (2005). A dinâmica das plantas daninhas e práticas de manejo. Londrina: Embrapa Soja, (Documentos, 260). P. 85. |