ABSTRACT
This study aimed to find an efficient method of inoculation of Stenocarpella maydis to produce white ear rot (WER) and estimate pathogen damage on maize grain yield components. Measured components were ear mass, grain mass per ear, and thousand grain weight. The experiments were performed in Ponta Grossa, PR, Brazil in a randomized block design with treatments arranged in a split plot with three replications. Three hybrids were studied. For each, five methods of inoculation in the ear at the soft dough stage were compared to un-inoculated controls. The ears were inoculated with 1 mL of the spore suspension (104 conidia/ml). Evaluations of the disease index (%), severity (%) and lesion area were performed in three (1st experiment) and four periods (2nd experiment), and the area under the disease progression curve was calculated for each of these periods. The area under the disease progression curve was calculated for each period. Inoculation at the center of the ear resulted in the best growth and development of the pathogen in both experiments. Inoculation at the base and center of the ears resulted in greater reductions in yield components, with degrees of damage varying from 27.8 to 43.1%. The inoculation of S. maydis in the center of the ear can be considered an appropriate method for resistance screening to WER in maize breeding programs.
Key words: White ear rot, disease progress curve, severity, yield components.
Fungal diseases (leaf, stalk, root and ear rot) can be observed during all stages of maize (Zea mays L.) crop development (Pereira et al., 2005). Maize ear rot diseases are of particular importance because they provide significant reductions in maize production (Duarte et al., 2009). Maize ear rot cause both weight reduction and quality losses in the grain. Some ear rot fungi also produce mycotoxins (Munkvold, 2003) that pose a health hazard to humans and animals consuming cereals products (Mukanga et al., 2010). Ears rots are one of the most dangerous foods and feed safety challenges to maize production in the world (Mesterházy et al., 2012).
The fungus Stenocarpella maydis (Berk.) Sutton [syn. Diplodia maydis (Berk.) Sacc.], causal agent of white ear rot (WER), occurs in practically every region of maize cultivation worldwide (Dorrance et al., 1998). S. maydis survival between seasons in residue of maize stalks, ears, and fallen grains. Conidia of the fungus are produced in fruiting structures called pycnidia, which are produced on infested maize residues. When pycnidia is opened the spores are spread by rain splash. In the silking, spores that are splashed up to the ear leaf and then deposited by rainwater around the ear shank have an opportunity to infect (Vincelli, 1997). In Brazil, changes in management practices, like less spacing between rows and monoculture and no-till systems enhance pathogen survival, and led to increases in inoculum quantity (Juliatti et al., 2007). In the Southern region of the country, the favorable environmental conditions promotes to WER development, with medium daily temperatures (25±2°C) and mild nights (12±2°C) resulting in higher incidence of this pathogen on maize crop (Pereira, 1995).
Because maize is the only commercial host of S. maydis, crop rotation is considered a viable method for its control (Costa Neto, 1976; Pinto et al., 1997; Reis and Mario, 2003; Reis et al., 2004; Pereira et al., 2005). However, a combination of crop sanitation, good agronomic practices and timely harvesting, has resulted in limited control of WER (Tembo et al., 2013). The utilization of resistant genotypes, therefore, could be a more effective control method, because can promote the field sanity and consequently inoculum reduction (Moremoholo et al., 2010).
Artificial selection strategies for genetic resistance to S. maydis could be achieved through breeding programs that aim to produce hybrids with greater resistance to WER. However, to identify resistant genotypes, inoculation methods must be developed to provide the same conditions as natural infection (Kuhnem Júnior et al., 2012). Currently, increasing numbers of maize breeding programs at both public and private institutions are initiating and expanding efforts to develop S. maydis resistant hybrids for both human and animal consumption (Mesterházy et al., 2012).
Finding the most appropriate and readily reproducible method of S. maydis inoculation will enhance the efficiency of maize breeding programs aimed at the selection of inbred lines and commercial hybrids with the highest level of resistance to this pathogen. The objective of this study was to identify efficient methods of S. maydis inoculation in the ears of maize hybrids and to estimate the damage caused by this pathogen by measuring losses in maize grain yield components.
Experimental environments
The experiments were conducted at the "Fazenda Escola Capão da Onça" of the State University of Ponta Grossa, Brazil, during the growing seasons of 2004/2005 and 2005/2006. The soil is classified as Red Yellow Latosol (Brazilian Soil Classification, Embrapa, 2006; and Oxisol, Ioamy, kaolinitic, thermic Typic Happludox, USDA Soil Taxonomy classification, 2006). The regional climate is classified as Cfb according to the Köppen classification, that is, subtropical humid mesothermal, with cool summers and severe and frequent freezing in the winter and with no defined dry season. The average annual temperature is 17.8°C and annual precipitation is 1,553 mm.
Experimental units consisted of 4 rows, 4.0 m in length with 0.8 m between rows and a seeding rate of 6 seeds per meter. In the experimental areas, no-till was the chosen cultivation system; 300 kg/ha of Nitrogen-Phosphorus-Potassium (NPK) (8-20-20) fertilizer was used, and manual seeding was performed with the help of jab-planters. In the first experiment, seeding occurred on 12/15/2004; seedling emergence on 12/22/2004; and nitrogenized fertilization was performed on the topsoil in a dosage of 200 kg/ha of urea when the plants were at the V2 stage (Fancelli, 1986). The second seeding was conducted on 11/27/2005, and the same cultivation practices used in the first experiment were followed, with seedling emergence on 12/03/2005.
Experimental design, plant material and inoculation methods
The experimental design was a randomized complete-block in split-plots, with three replications. The hybrids studied in the main plots were provided by the company Dow AgroSciences (8420, 8480 and 2B710), and the effect of ear inoculation: (1) in the rachis, (2) at the base, (3) in the middle, (4) at the tip, (5) sprayed on the style/stigma, and (6) not inoculated (absolute control) were studied in the subplots.
The isolated strains of S. maydis were given by Dow AgroSciences. Five discs of a S. maydis colony, 5 mm in diameter, were transferred to flasks containing sorghum grains (Silva and Juliatti, 2005) doubly autoclaved at 121°C for 20 min with a 24 h interval between autoclaving according to Mario et al. (2011). The cultures were incubated in a growth chamber at 25 ± 2°C with a photoperiod of 12 h light/12 h dark for 15 days until spores were produced. The inoculum was prepared by adding 200 ml of sterile distilled water to each flask, followed by stirring for the release and formation of a conidial suspension. The spore suspension was filtered through a double layer of cheesecloth, and its concentration was adjusted to 104 conidia mL-1. Inoculation was performed with 1 ml of conidial suspension in different parts of the ear with a veterinary-use automatic syringe (50 ml) or sprayed on the style/stigma of the ear with a hand sprayer. The ears were inoculated at the soft dough stage (R4) on 03/24/2005 and 03/15/2006. At inoculations the environment conditions were considered ideals for pathogen development, with medium temperature around 23°C and high relative humidity (> 80%) for both experiments.
Disease severity and estimate damage
To evaluate the growth/colonization of the pathogen on the hybrid ears, three evaluations were conducted in the 2004/2005 season on 04/05/2005, 04/15/2005, and 04/26/2005, and four evaluations in the 2005/2006 season on 03/29/2006, 04/10/2006, 04/29/2006, and 05/20/2006. For each evaluation, five ears were sampled from the central lines of each sub-plot in the experiments. To assess the severity of the white ear rot, diagrammatic scales and notes were used according to the methodology proposed by Azevedo (1997). Additionally, the length and diameter of the lesions in each ear were measured using a tape measure (cm). The last evaluation of the experiments consisted of the determination of the yield components: ear mass (g), grain mass per ear (g), and thousand grain weight (g). Damage (%) was estimated for these characteristics by comparing the damage encountered in each inoculation method to the damage in the control treatment (without inoculation).
The severity grades were transformed into percentages, representing the Disease Index (DI), through the equation proposed by McKinney (1925), as follows:
Statistical analysis
The area under the disease progress curve (AUDPC) was calculated for the disease index (%), severity data (%), and lesion area of each evaluation according to the equation proposed by Shaner and Finney (1977). The data collected in the experiments were subjected to analysis of variance using the statistical program SISVAR (Ferreira, 2011), and the treatment means, when significantly different, were compared using the Tukey test at 5% probability.
The S. maydis strains obtained from Dow AgroSciences were highly aggressive and allowed the intense growth of white ear rot in the three maize hybrids studied. For both experiments, the AUDPC values for the disease index (DI), severity, and lesion area of WER showed highly significant differences (p < 0.01) among the different pathogen inoculation methods. The interaction of the inoculation methods and maize hybrids only demonstrated statistical significance (p < 0.05) for the lesion area in both experiments. The lack of a significant effect of the hybrid on the AUDPC characteristics (DI and severity) in the 2004/2005 season may be related to dent grain texture of the hybrids used in this experiment. Mario et al. (2003), evaluating the reaction of six hybrids to WER, found that the hybrids with a dent type grain texture had the lowest incidence of S. maydis. The authors also found that the hybrids with dent grain type characteristics produced the highest yields, while the hybrids with a flint grain type had the lowest yields.
Inoculation at the ear rachis and style/stigma spraying were not efficient in the 2004/2005 season, as similar for the non-inoculated control treatment (Table 1). The inoculation of S. maydis in the middle of the ear gave the highest disease severity, followed by inoculation at the base of the ear; the disease index was significantly lower when the inoculation was performed at the tip of the ear. Likewise, when the AUDPC was evaluated for severity of the pathogen, inoculation in the middle of the ear proved to be better than the other methods for evaluation of disease (Table 1).
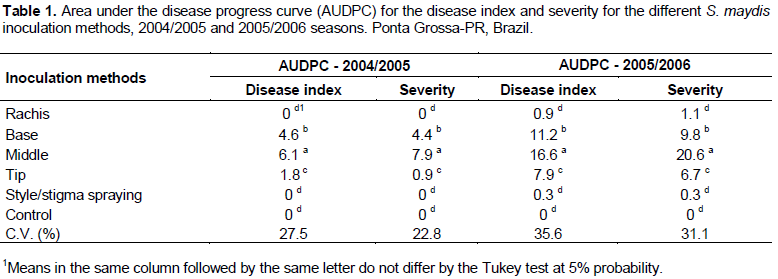
Similar to the results observed in the 2004/2005 season, the AUDPC disease and severity indices were not significantly different for the plants inoculated at the ear rachis and style/stigma spray and the non-inoculated control plants in the 2005/2006 season (Table 1). A significant effect was observed for the interaction of the inoculation methods with the maize hybrids for the AUDPC lesion area in the 2004/2005 and 2005/2006 seasons (Table 2), indicating different behavior of the hybrids in response to different pathogen inoculation methods. For all hybrids, the inoculation of S. maydis in the middle and base of the ear led to the largest lesion area, followed by inoculation at the tip of the ear. In both experiments, pathogen inoculation by spraying the conidial suspension on the ear style/stigma appeared to have no effect on any of the evaluated characteristics (Table 2).
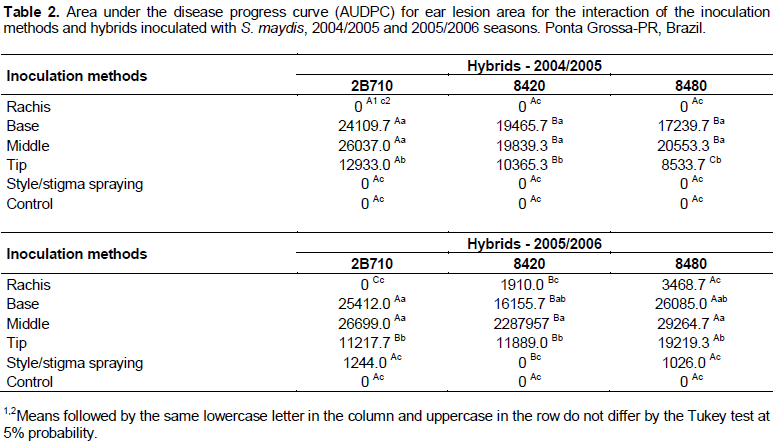
Pathogen inoculation by injection of the rachis of the ear did not result in the efficient growth/development of WER in either of the two seasons, with results that were not significantly different from the results of the non-inoculated control. This trend towards decreased development of the pathogen was also observed by Mario et al. (2003) in the method consisting of the deposition of the spore suspension in the leaf sheath behind the ear; in contrast, Bensch et al. (1992) observed a higher incidence of WER through this method of inoculation. Klapproth and Hawk (1991) obtained higher infection levels with the injection of S. maydis inside the ear; these results, similarly, showed that the inoculation at the middle and the base of the ear led to the highest levels of pathogen growth. Flett and McLaren (1994) determined the great potential diseases in different maize hybrids inoculating into the apical whorl 2 weeks prior anthesis.
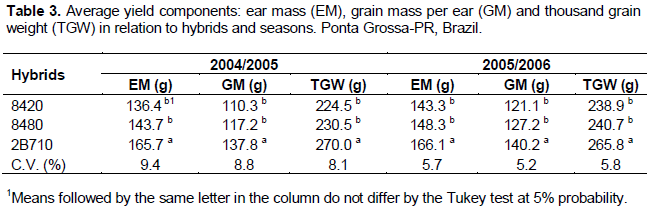
Recently Mario et al. (2011) studied three methods of inoculation with S. maydis (natural, spray on stigmas, and deposition on the peduncle) and observed infection rates of 21.0, 39.8, and 44.3% in the ears, respectively. The authors concluded that the inoculation methods consisting of stigma spraying and deposition on the peduncle improved upon the field deposition method of applying the conidial suspension and allowed susceptible hybrids to be differentiated from resistant ones. For the grain yield components (Table 3), highly significant effects of both the hybrids and the inoculation methods (p < 0.01) were observed in both seasons for the ear mass (EM), grain mass per ear (GM), and thousand grain weight (TGW). The differences observed in the yield components among the hybrids in both seasons indicate the different yield potentials and genetic backgrounds of the hybrids, with hybrid 2B710 achieving the most positive results.
In both experiments, the inoculation at the middle and base of the ear resulted in the largest reduction in the ear mass, ear grain mass and thousand grain weight (Table 4). The inoculation at the ear rachis and style/stigma spraying resulted in the same yield results as were seen in the control treatment (without inoculation); that is, they did not affect the reduction of the grain yield components (Table 4). These results demonstrate a direct connection between the extent of the disease (disease index, severity and lesion area) and the method of S. maydis inoculation. Overall, the inoculation at the middle and base of the ears resulted in the highest pathogen colonization and consequent significant reduction of yield components, whereas the inoculation at the tip of the ear and style/stigma spray resulted in lower colonization of the ears and thus limited damage to the production components. Silva et al. (2005) reported that S. maydis inoculation with a toothpick in the middle of the ear, followed by injection at the base of the ear (R2 stage) provided the greatest AUDPC for this disease; however, inoculation in the middle ear with a toothpick was the only method that resulted in significantly reduced grain yield.
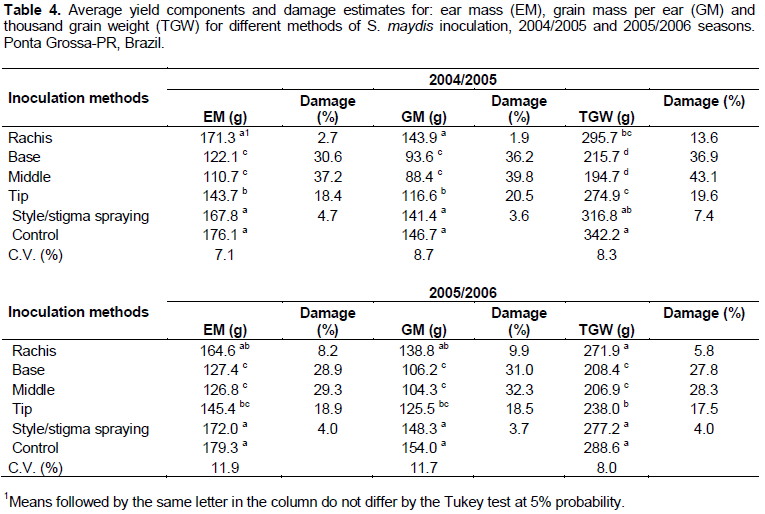
The ear mass was severely affected by the inoculation both at the middle and at the base of the ear. The middle ear inoculation method led to decreases in the ear mass of 37.2 and 29.3% in the first and second seasons, respectively (Table 4). The inoculation at the base of the ear resulted in 30.6 and 28.9% of damage reduction in the first and second experiments, respectively. The rachis and style/stigma spraying inoculation methods resulted in significantly reduced damage estimates, with damage rates between 2.7 and 8.2% for the inoculation in the ear rachis. These estimates may reflect the low amount of disease (DI, severity, and area of injury) associated with S. maydis inoculation in the rachis and style/stigma spraying (Table 4). Mario et al. (2003) reported that spraying the conidial suspension on the style/stigma of the ear caused significant reductions in the average grain yield of the inoculated hybrids; these results differ from the results obtained in our study for style/stigma spraying.
The results of both experiments confirmed the negative effect of the pathogen on the ear grain mass, particularly when the inoculation occurred at the middle and at the base of the ear. For the first experiment, while an average grain weight of 146.7 g per ear was achieved in the control treatment, the inoculation at the middle of the ear resulted in a mass of only 88.4 g (-39.8%). Likewise, the damage reduction in the second season was estimated at 32.3% for the grain weight (Table 4). Additionally, a significant reduction in TGW was observed for the plants inoculated at the middle or at the base of the ear, with the damage estimates ranging from 27.8 (base) to 43.1% (middle) (Table 4).
The hybrids used in our experiments (8420, 8480 and 2B710) have grain textures ranging from semi-dent to flint. In a study by Mario et al. (2003), the hybrids obtaining the highest yields and the lowest incidences of infection with S. maydis were dent grain characteristics, while the hybrids with a flint grain were the most affected by the pathogen (greater incidence and severity), with a significant reduction in the grain yield.
The results obtained in this study emphasize the vulnerability of maize ears to S. maydis infection at the soft dough stage (R4). In fact, the soft dough stage is the ideal period for differentiating susceptible from resistant germplasms (Chambers, 1988) and also an auxiliary tool in determining the disease potentials (Flett and Mclaren, 1994). Thus, significant reductions in the measures of yield are associated with inoculation methods that allow further growth of the lesion, including inoculation at the middle and the base of the ear. It is important to identify the most effective method of inoculating S. maydis so that high levels of resistance to the pathogen can be selected in inbred lines and hybrids. The results obtained in this study indicate that inoculation at the middle and the base of the ear are the most promising methods for identifying variants with resistance/susceptibility to this pathogen.
The authors have not declared any conflict of interest.
REFERENCES
|
Azevedo LS (1997). Manual de quantificação de doenças de plantas. Ed. do autor. P. 114. |
|
|
Bensch MJ, Van Staden J, Rijkenberg FHJ (1992). Time and site of inoculation of maize for optimum infection of ears by Stenocarpella maydis. J. Phytopathol. 136:265-269.
CrossRef |
|
|
Chambers KR (1988). Effect of time of inoculation on Diplodia stalk and ear rot of maize in South Africa. Plant Dis. 72(6):529-531.
CrossRef |
|
|
|
Costa Neto JP (1976). Lista de fungos sobre graminae (capins e cereais) no Rio Grande do Sul. Revista da Faculdade de Agronomia 1(2):43-78. |
|
|
Dorrance AE, Hinkelman KH, Warren HL (1998). Diallel analysis of Diploida ear rot resistance in maize. Plant Dis. 82(6):699-703.
CrossRef |
|
|
|
Duarte RT, Juliatti FC, Freitas PT (2009). Eficácia de diferentes fungicidas na cultura do milho. Biosci. J. 25(4):101-111. |
|
|
|
Embrapa (2006) Sistema brasileiro de classificação de solos. Centro Nacional de Pesquisa de Solos. Brasília: Embrapa Produção de Informação; Rio de Janeiro: Embrapa Solos. P. 305. |
|
|
|
Fancelli AL (1986). Plantas alimentícias: guia para aula, estudos e discussão. Centro Acadêmico "Luiz de Queiroz". ESALQ/USP. P. 131. |
|
|
|
Ferreira DF (2011). Sisvar: a computer statistical analysis system. Ci. Agrotec. (UFLA) 35(6):1039-1042. |
|
|
Flett BC, McLaren NW (1994). Optimum disease potential for evaluating resistance to Stenocarpella maydis ear rot in corn hybrids. Plant Dis. 78:587-589.
CrossRef |
|
|
|
Juliatti FC, Zuza JMLF, Souza PP, Polizel AC (2007). Efeito do genótipo de milho e da aplicação foliar de fungicidas na incidência de grãos ardidos de milho. Biosci. J. 23(2):34-41. |
|
|
Klapproth CJ, Hawk AJ (1991). Evaluation of four inoculation techniques for infecting corn ears with Stenocarpella maydis. Plant Dis. 75(10):1057-1060.
CrossRef |
|
|
|
Kuhnem Jr PR, Casa RT, Bogo A, Agostineto L, Bolzan JM, Miqueluti DJ (2012). Effects of temperature, light regime and substrates on the production and germination of Stenocarpella maydis pycnidiospores. Acta Sci. 34(1):11-16. |
|
|
Mário JL, Reis EM, Bonato ER (2003). Reação de híbridos de milho à podridão branca da espiga. Fitopatol. Bras. 28(2):155-158.
CrossRef |
|
|
|
Mário JL, Reis, EM, Juliatti FC (2011). Three inoculation methods for screening corn germplasm to white ear rot resistance. Trop. Plant Pathol. 36(6):362-366. |
|
|
|
McKinney HH (1925). Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 26(9):195-219. |
|
|
Mesterházy Á, Lemmens M, Reid LM (2012). Breeding for resistance to ear rots caused by Fusarium spp. in maize – a review. Plant Breed. 131(1):1-19.
CrossRef |
|
|
Moremoholo L, Shimelis H, Mashela PW (2010). Yield response and Stenocarpella ear rot reaction among selected maize inbred lines and top cross hybrids. Euphytica 174:231-238.
CrossRef |
|
|
Mukanga M, Derera J, Tongoona P, Laing MD (2010). A survey of pre-harvest ear rot diseases of maize and associated mycotoxins in south and central Zambia. Int. J. Food Microbiol. 141(3):213-221.
CrossRef |
|
|
Munkvold GP (2003). Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 41:99-116.
CrossRef |
|
|
|
Pereira OAP (1995) Situação atual de doenças da cultura do milho no Brasil e estratégias de controle. In: Resistência Genética de Plantas a Doenças. Piracicaba SP. ESALQ-USP. |
|
|
|
Pereira OAP, Carvalho RV, Camargo LEA (2005). Doenças do milho (Zea mays L.). In: Kimati H, Amorim L, Rezende JAM, Bergamin Filho A, Camargo LEA (Eds.) Manual de Fitopatologia. Doenças das Plantas Cultivadas. 4ª. Ed. São Paulo, SP. Ceres 2:477-488. |
|
|
|
Pinto NFJA, Fernandes FT, Oliveira E (1997). Controle de doenças do milho. In: Vale FXR, Zambolim L (Ed.). Controle de doenças de plantas: grandes culturas. Imprensa Universitária, UFV, Viçosa, MG. pp. 821-864. |
|
|
|
Reis EM, Casa RT, Bresolin ACR (2004). Manual de diagnose e controle de doenças do milho. 2ª Ed. Lages, SC. Graphel. |
|
|
Reis ME, Mario JL (2003). Quantificação de inóculo de D. maydis e D. macrospora em restos culturais, no ar e sua relação com a infecção em grãos de milho. Fitopatol. Bras. 28(2):143-147.
CrossRef |
|
|
Shaner G, Finney RE (1977). The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67:1051-1056.
CrossRef |
|
|
|
Silva AR, Juliatti FC (2005). Esporulação de Diplodia maydis e Diplodia macrospora em diferentes meios de cultura. Biosci. J. 21(3):127-131. |
|
|
|
Silva AR, Juliatti FC, Brito CH, Gomes LS (2005). Métodos de inoculação de Stenocarpella maydis em três populações de milho. Summa Phytopathol. 31(1):79-83. |
|
|
Tembo L, Asea G, Gibson PT, Okori P (2013). Resistance breeding strategy for Stenocarpella maydis and Fusarium graminearum cob rots in tropical maize. Plant Breed. 132:83-89.
CrossRef |
|
|
|
USDA Soil Taxonomy classification (2006). Keys to soil taxonomy. 10th. Ed. Washington, USDA Natural Resources Conservation Service, US Govt. Printing Office. |
|
|
|
Vincelli P (1997). Ear rot of corn caused by Stenocarpella maydis (= Diplodia maydis). Cooperative extension service, University of Kentucky College of Agriculture. 43:3. |