ABSTRACT
The study was aimed at evaluating the growth of eucalyptus seedlings irrigated with saline water. It was conducted on full-sun bench in the Goiás State University. The experiment was set up following the completely randomized design, with five treatments (plants irrigated daily with water with an electrical conductivity equal to 0; 2; 4; 6 and 8 dS m-1) and six replications. The eucalyptus plants showed small alterations of the growth variables (height, diameter and biomass). Vigorous vegetative growth and retention of tissue moisture indicate that eucalyptus is tolerant to salinity.
Key words: Tolerance, electrical conductivity, biomass
The increase in greenhouse gas concentrations in the Earth's atmosphere, resulting from anthropic actions, has been jeopardizing natural resources and the survival of mankind. The constant pressure for sustainable development and preservation of natural resources entails the necessity of exploiting planted forests.
The constant increase in the economic value and shortage of high-quality timber intensified the multiple use of the genus Eucalyptus (Souza et al., 2012). In Brazil, the genus Eucalyptus found ideal growth conditions. Plantation productivity in Brazilian lands is higher than that of traditional countries like Australia (origin of the species).
Brazil has great potential for exploitation of planted forests in most of its territorial extent, as a result of its climate and soil conditions, biodiversity, land and manpower availability, as well as documented technical competence in the field of forestry. The competitiveness of the Brazilian forest sector, which stems from the climatic conditions and from the technology developed by companies and research institutions, places the country at a unique position in the international scene (Ferreira et al., 2012). The Brazilian forestry sector accounts for 3.5% of the gross domestic product (Abraf, 2013). Currently, planted forests in Brazil comprise about 6.9 million hectares, 4.9 million of which with eucalyptus (about 25% of the world plantation) and 1.6 million with pine (Gonçalves et al., 2013).
Despite its high potential in the forestry sector, Brazil can produce even more and transfer wealth to other economy segments. The increased interest in the use of eucalyptus for diverse purposes, as timber or otherwise and for the recovery of degraded areas has expanded the species exploitation. Population growth increases the demand for timber and cultivation areas, requiring the exploitation of marginal areas (Lopes and Klar, 2009). Eucalyptus is quite adaptable to different conditions of heat, light and dryness. However, production can be better ensured by the use of irrigation, and even the possibility of cultivating it in salinity conditions should be researched (Veras et al., 2011). The quality of the water from many sources is low, especially water from wells and surface reservoirs. Since the water used for periodic irrigation contains soluble salts, it causes incorporation of salts into the soil profile. Without leaching, salt settles in the root zone and on the soil surface as a result of water evaporation (Veras et al., 2011).
Salinity in soils of arid and semi-arid regions is an issue of social concern, since salts affect millions of hectares in the whole world, which reduces the capacity for production in these areas (Lima et al., 2006). Both in soils and waters salinity is one of the main causes of crop yield reductions (Flowers, 2004; Matos et al., 2013) due to the osmotic, toxic or nutritional effect (Viana et al., 2004). Salinity affects about 10% of the Earth’s surface. According to Shannon et al. (1994), water electrical conductivity equal to or higher than 4 dS m-1 drastically reduces agricultural productivity. Globally, the production in about 400 million hectares of land used for agriculture is severely limited by salinity (Bot et al., 2000).
According to Lopes et al. (2012), the Eucalyptus platyphylla plants are tolerant to irrigation water salinity, as they present small variation in diameter and plant height when irrigated with low quality water. However, little is known about the tolerance of Eucalyptus plants to salinity. In view of the need for such information for the production of seedlings for field planting, and for better understanding the attributes for salinity tolerance, which would allow for commercial exploitation with saline water irrigation, the present study is aimed at evaluating the cultivation of eucalyptus seedlings irrigated with saline water.
Experimental design
The study was conducted on a transparent cover bench, located in the State University of Goiás, Ipameri Unit (Lat. 17° 43' 19'' S, Long. 48° 09' 35'' W, Alt. 773 m), Ipameri, Goiás. According to the Köppen classification, this region has Aw-type climate.
The experiment was conducted following the completely randomized design, with five treatments (plants irrigated daily with water with electrical conductivity (ECw) equal to 0, 2, 4, 6 and 8 dS m-1) and six replications. Seedlings of Eucalyptus urophylla aged 100 days were planted in pots containing 8 L of a substrate composed of red-yellow oxisol, sand and manure at ratios of 3: 1: 0.5, respectively. The chemical analysis of the mixture showed the following values: pH 6.4; 19 g dm-3 of NOM; 2.4 mg dm-3 of P, 109 cmolc dm-3 of K, 1.5 cmolc dm-3 of H+Al, 3.2 cmolc dm-3 of Ca, 1.6 cmolc dm-3 of Mg, 27.7 mg dm-3 of Zn, 77.20% of BS, and 6.58 of CEC. After analysis of the mixture composition, fertilization and pH correction of the substrate were performed according to technical recommendations for eucalyptus cultivation. The seedlings were irrigated for 30 days with a solution volume corresponding to the daily evapotranspiration. The choice for Eucalyptus urophylla was due to its high commercial exploitation in Brazil and use for various purposes, resistance to eucalyptus cancer (Cryphonectria cubensis), high plasticity and adaptation to diverse ecosystems. The solution volume supplied to the plant was calculated according to recommendations of Allen et al. (2006). NaCl was added to the local water supply, in order to obtain solutions with different electrical conductivity, the quantity (Q) being determined by the equation Q (mg L-1) = 640 × ECw (dS m-1), according to Rhoades et al. (2000), where ECw represents the desired electrical conductivity value of the water. Subsequently, the conductivity values were checked and verified with a conductivity meter.
At 130 days and 30 days after treatment, the following variables were analyzed: Number of leaves, plant height, stem diameter, relative water content (RWC), total chlorophyll and carotenoid contents, root mass ratio (RMR), stem mass ratio (SMR), leaf mass ratio (LMR), transpiration and total biomass.
Growth variables
The plant height and stem diameter were measured using a graduated ruler and a digital pachymeter. The number of leaves was obtained by counting all the plant leaves. The destructive analyses were carried out next, after leaves, roots and stems were separated, put to dry in an oven at 72°C until they turned into a steady dry mass, and finally weighed. With the dry mass data, the leaf mass ratio (LMR), root mass ratio (RMR), stem mass ratio (SMR), leaf foliar area ratio (LAR) and total biomass were calculated.
Relative water content in leaf (RWC)
In order to determine the relative water content, ten 12 mm leaf disks were extracted, then weighed and placed for four hours in Petri dishes with distilled water to saturate. After that, the disks were weighed again and put to dry at 70°C for 72 h, the dry weight in grams being obtained afterwards.
Transpiration
The daily transpiration was estimated through gravimetry, by measuring the difference in the pots weight at one-hour intervals between 07:00 and 18:00 hours, as described by Cavatte et al. (2012).
Photosynthetic pigments
Leaf disks of known area were collected and placed in jars containing dimethyl sulfoxide (DMSO), to determine the total carotenoid and chlorophyll concentrations. Later, the extraction was carried out in water bath at 65°C for one hour. Aliquots were removed for spectrophotometric reading at 480, 649 and 665 nm.
The chlorophyll a (Cl a), chlorophyll b (Cl b) and carotenoid (Car) contents were determined following the equation proposed by Wellburn (1994).
Data analysis
The experiment followed the completely randomized design with five treatments and six replications. The data were submitted to variance analysis and then to regression analysis using SISVAR 5.3 software (Ferreira, 2011).
During the experiment period, the temperature varied between 12 and 30°C and the relative air humidity between 50 and 80%. On the evaluation day, the average air temperature was 30°C and the relative humidity 65%. The variance analysis and the averages of all analyzed variables can be found in Tables 1 and 2. Regardless of the electrical conductivity of the irrigation water (ECw), the plant height, stem diameter and leaf water content showed non-significant variation. However, the RWC showed a 16% decrease when the treatment with deionized water irrigation was compared to the treatment using water with ECw of 8 dS m-1. The number of leaves, branches and level of transpiration decreased as the ECw increased, showing a significant statistical difference. The highest variation in the number of leaves was 42% when comparing the treatment with deionized water irrigation to the treatment using water with ECw of 8 dS m-1. The number of branches was 30% lower in plants irrigated with ECw of 8 dS m-1 in comparison with the control irrigated with deionized water. The total plant transpiration was 68% lower in plants irrigated with ECw of 8 dS m-1 in comparison with the control.
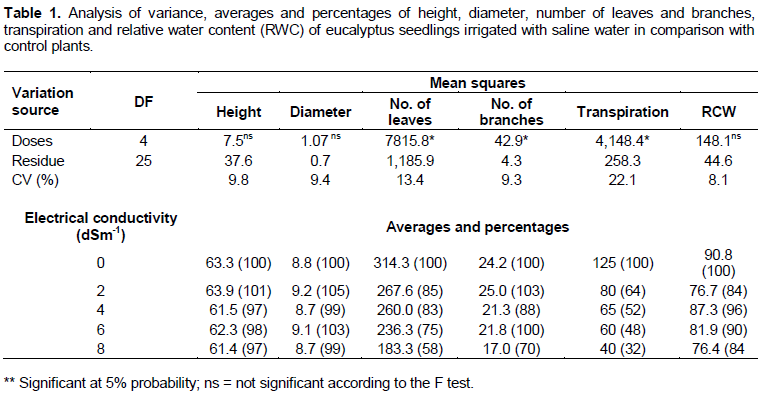
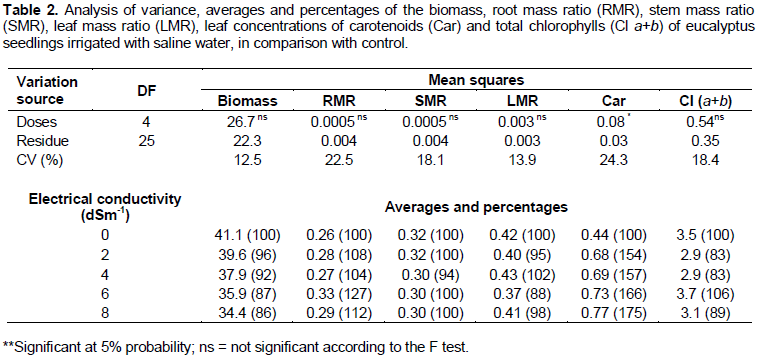
Regardless of the electrical conductivity of the irrigation water (ECw), the biomass, root mass ratio, stem mass ratio, leaf mass ratio and total chlorophyll contain little variation (Table 2). However, even without statistical difference, it can be observed that the biomass showed a 14% decrease when the deionized water irrigation treatment is compared to the irrigation treatment using water with ECw of 8 dS m-1. The foliar concentration of total carotenoids was 75% lower in the control plants irrigated with deionized water than in the plants irrigated with ECw of 8 dS m-1.
The variables number of leaves, number of branches, transpiration and foliar concentration of carotenoids showed significance at the 5% probability level in F test and, therefore, regression was carried out for each one as shown in Figure 1.
Under natural conditions, abiotic stress can cause irreversible damage to plants. And tolerance to salinity can represent an expansion of the agricultural frontier to obtain economic payback in areas that used to be inadequate. High adaptability to climate and soil variation, combined with steady growth under stress conditions (Pinto et al., 2008), may have helped the eucalyptus to tolerate the salinity of the irrigation water with little alteration in growth variables, as discussed subsequently.
Regardless of the ECw, eucalyptus plants showed an intense vegetative growth that resulted in similar biomass accumulation among treated plants. The increase in electrical conductivity of the irrigation water and consequent reduced potential of the solution were not sufficient to generate significant alterations to growth variables (height, diameter and biomass). The results confirm those of Lopes et al. (2012) when evaluating the growth of eucalyptus irrigated with saline water. The sustained growth under the conditions imposed represents an important sign of tolerance to salinity (Matos et al., 2013). The eucalyptus plant may have an efficient mechanism for soil water extraction, since even under conditions of low water supply; it manages to extract enough water from the soil to sustain growth.
The electrical conductivity variation in the irrigation water did not cause any significant alteration in the distribution of biomass to the root system, stem and leaf (RMR, SMR and LMR). The physiological drought resulting from the reduction in the osmotic potential is a direct effect of saline stress. Under such conditions, plants subjected to high electrical conductivity allocate the little existing biomass to the development of the root system, since vigorous and deep roots have higher potential of extracting water and nutrients from the soil. Greater biomass distribution to the root system under reduced osmotic and water supply conditions are a common response of various species (Góes et al., 2009; Matos et al., 2013).
The common response of greater growth of the root system under water restriction conditions was not found in this study, which shows that saline stress did not limit water availability to the eucalyptus plants to the point that it would induce more root growth. The reduced variation in growth of the root system of eucalyptus plants irrigated with saline water has been documented and seems to be common in irrigations with water electrical conductivity lower than 8 dS m-1 (Lopes et al., 2012; Feikema and Baker, 2011).
Reduction in the number of leaves, small variation of the relative water content and reduction in transpiration show that eucalyptus slows down dehydration as a strategy to endure drought. For this purpose, the plant reduces water loss by possessing small transpiring leaf area, thus keeping itself hydrated for a longer period. Water shortage induced by the osmotic effect, which is characteristic of the seasonal drought, causes morphological and anatomical alterations in the plants that unbalance water absorption and transpiration. Among the morphological changes, the most significant one is the reduction in the number and size of the leaves (Santana et al., 2011). Sprouting and early development of the leaves depend on the water supply to the plant. Shorter water availability to irrigated plants with high ECw resulted in lesser water availability for the plant metabolism, such as formation and development of new leaves. Reducing the number of leaves is an important strategy to endure the soil low water supply, as it will reduce the transpiration area. Slowing down dehydration allows for immediate survival. Adjusting the total leaf area and the number of leaves is necessary to bear the imposed stress and to adapt to the new climatic conditions (Matos et al., 2013). Retaining leaf hydration may also be associated to an efficient mechanism for the species stomatal control, since the transpiration rate is reduced with the increase in ECw.
The lower number of branches in plants treated with saline water without significant alteration in the stem mass shows that eucalyptus in salinity condition gives priority to the main stem, possibly to facilitate the hydraulic conductivity of the soil solution along the xylem.
The reduction in transpiration because of lesser stomatic conductivity may give rise to a photosynthesis disorder as a result of lesser CO2 influx and increase in leaf temperature. In such circumstances, photosynthesis inhibition and oxidative stress with damage to proteins and membranes are common. Visual symptoms of oxidative stress were sporadically seen in some leaves of plants irrigated with ECw higher than 2 dS m-1. We suggest that the photoprotection of the eucalyptus plants occurred as a result of the higher leaf concentration of total carotenoids in plants irrigated with high ECw. The small variation in chlorophyll leaf concentration is a sign of lack of toxicity caused by salt.
The minor changes in growth variables combined with a strategy of slow dehydration and a photoprotection mechanism indicates that eucalyptus plants are tolerant to salinity. However, further studies with different salinity levels of irrigation water for longer periods of time are required to evaluate the accumulation of salts in the soil and the toxicity of salts in long-term field irrigation before the use of saline water in commercial eucalyptus plantations is recommended.
1. Eucalyptus plants show vigorous vegetative growth when irrigated with saline water with electrical conductivity lower than 8 dS m-1.
2. Eucalyptus plants slow down dehydration reducing the number of leaves and the transpiration level.
3. Eucalyptus plants are tolerant to salinity.
The authors have not declared any conflict of interest.
State University of Goiás and improving coordination of senior staff (CAPES).
REFERENCES
|
ABRAF (2013). Associação brasileira de produtores de florestas plantadas (ABRAF). |
|
|
Allen RG, Pruit WO, Wright JL, Howell TA, Ventura F, Snyder R, Itenfisu D, Steduto P, Berengena J, Yrisarry JB, Smith M, Pereira LS, Raes D, Perrier A, Alves I, Walter I, Elliott RA (2006). recommendation on standardized surface resistance for hourly calculation of reference ETo by the FAO56 Penman-Monteith method. Agric. Water Manage. Amsterdam 81:1-22.
Crossref |
|
|
|
Bot A, Nachtergaele F, Young A (2000). Land resource potential and constraints at regional and country levels. World Soil Resources Report, FAO. 90:122. |
|
|
Cavatte PC, Oliveira AAG, Morais LE, Martins SCV, Sanglard LMVP, Damatta FM (2012). Could shading reduce the negative impacts of drought on coffee? The morphophysiological analysis. Physiol. Plantarum Copenhagen 144:111–122.
Crossref |
|
|
Feikema PM, Baker TG (2011). Effect of soil salinity on growth of irrigated plantation Eucalyptus in south-eastern Australia. Agric. Water Manage. 98:1180-1188.
Crossref |
|
|
|
Ferreira DF (2011). Sisvar: a computer statistical analysis system. Ciênc. Agrotecnol. 35:1039-1042. |
|
|
Ferreira SM, Petrauski C, Marques GM, Silva ML, Cordeiro AS, Soares NS (2012). Competitividade do Brasil no mercado internacional de madeira serrada. Cerne 18:99-104.
Crossref |
|
|
Flowers TJ (2004). Improving crop salt tolerance. J. Exper. bot. 55:307-319.
Crossref |
|
|
|
Góes GB, Dantas DJ, Mendonça V, Araújo WBM, Freitas PSC, Medeiros LF (2009). Crescimento inicial de muda tipo pé-franco de tamarindeiro (Tamarindus indica L.) em diferentes níveis de salinidade na água. Agrarian 2:63-70. |
|
|
|
Gonçalves JLM, Alvares CA, Higa AR, Silva LD, Alfenas AC, Stahl J, Ferraz SFB, Lima WP, Brancalion PHS, Hubner A, Bouillet J-PD, Laclau J-P, Nouvellon Y, Epron D (2013). Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manage. 23:222-235. |
|
|
|
Lopes TC, Klar AE (2009). Influência de diferentes níveis de salinidade sobre aspectos morfofisiológicos de mudas de Eucalyptus urograndis. Rev. Irrig. 14:68-75. |
|
|
|
Lima MDB, Bull LT, Grassi Filho H (2006). Índices fisiológicos e absorção de nutrientes pela cultura da cebola submetida a condições de salinidade e estresse hídrico. Irrigation 3:356-366. |
|
|
Lopes TC, Lima KB, Klar AE (2012). Desenvolvimento inicial de plantas de Eucalyptus platyphylla submetidas a níveis de salinidade. Irrigation 17:494-500.
Crossref |
|
|
Matos FS, Rocha EC, Cruvinel CKL, Ribeiro RA, Ribeiro RP, Tinoco CF (2013). Desenvolvimento de mudas de pinhão-manso irrigadas com água salina. Rev. Bras. Ciênc. Solo 37:947-954.
Crossref |
|
|
|
Pinto Júnior JE, Garlipp RCD (2008). Eucalipto. In: Albuquerque ACS, Silva AG, Agricultura tropical: quatro décadas de inovações tecnológicas, institucionais e políticas. Brasília, DF: Embrapa Inform. Tecnol. 1:801-822. |
|
|
|
Rhoades JD, Kandiah AM, Marshali AM (2000). Uso de águas salinas para produção agrícola. Estudos da FAO - Irrigação e Drenagem, Universidade Federal da Paraíba 48:1-117. |
|
|
Santana MJ, Carvalho JÁ, Silva EL, Miguel DS (2011). Efeito da irrigação com água salina em um solo cultivado com o feijoeiro (Phaseolus vulgaris L.). Ciênc. Agrotecnol. 27:443-450.
Crossref |
|
|
|
Shannon MC, Crieve CM, Francois LE (1994). Whole Plant Response to Salinity. In: Wilkiman RE (Ed.) Plant Environment Interactions. New York: Marcel Dekker pp. 199-244. |
|
|
Souza JT, Trevisan R, Denardi L, Stangerlin DM, Vivian MA, Haselein CR, Santini JE (2012). Qualidade da madeira serrada provenientes de árvores dominantes e média de Eucalyptus grandis submetidos a secagem. Cerne 18:167-174.
Crossref |
|
|
Veras RP, Laime EMO, Fernandes PD, Soares FAL, Freire EA (2011). Altura de planta, diâmetro caulinar e produção do pinhão-manso irrigado sob diferentes níveis de salinidade. Rev. Bras. Engenharia Agríc. Ambiental 15:582–587.
Crossref |
|
|
Viana SBA, Fernandes PD, Gheyi HR, Soares FAL, Carneiro PT (2004). Índices morfofisiológicos e de produção de alface sob estresse salino. Rev. Bras. Engenharia Agríc. Ambiental. 8:23-30.
Crossref |
|
|
Wellburn AR (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144:307-313.
Crossref |