Soil insects are a major source of crop loss in tropical, subtropical and temperate zones. Many soil insecticides are either/or highly toxic to humans, have serious other non-target effects, or are a banned from use nationally or internationally. An alternative strategy is the use of beneficial (entomopathogenic) nematodes as they are well-adapted to the soil environment and non-toxic. However, the establishment of locally adapted mass production systems for beneficial nematodes requires considerable technological development effort and time. We therefore analyzed the design of 16 in-vitro semi-solid, 3 in-vivo and 2 combined in-vitro + in-vivo nematode production factories from Rwanda, Switzerland, PR China, DPR Korea, to provide an understanding of the rationale behind factory site selection, as well as external and internal factory designs. The factories should, regardless of the production method, consist of at least six rooms in one building. Their allocation depends on the work-flow, insulation of cultures and storage, as well as on separations to avoid cross-contaminations. Our findings propose optimal standard designs for nematode mass production factories and give insight into steps for planning their establishment. This information will be vital to support the dissemination of such technologies to other locations in-country, or to new countries, with the ultimate aim to more safely control soil pests.
In farmland across the world, soil insect pests are at least as significant in terms of economic damage as the above-ground pests (Edwards, 1976;
Toepfer et al., 2014). Soil insects mainly cause yield losses due to their damaging effects on the early developmental stages of crops, such as seeds and seedlings, or on young trees or bushes (
Edwards, 1976;
He, 2007;
Nazarenjo et al., 2001). Soil insects can also attack to-be-harvested below ground plant parts, such as grubs or weevils in tuber crops (Lebot, 2009), or dipteran larvae in allium and brassica vegetables (Pan and Xia, 1993), often followed by secondary pathogen infections. In this case, the harvest is directly affected and economic impacts are higher than for the damage on early crop stages.
Unfortunately, soil insect pests are difficult to control because of their hidden nature in the soil, combined with the limited knowledge on their life cycles and below-ground ecology. There are several other reasons for the difficulty of solving agricultural problems related to soil pests. The increasingly frequent continuous strip cropping of the same combination of vegetables or vegetables with field crops in many regions globally appears to favor the survival of certain soil pest populations (
Chen et al., 2004;
Toepfer et al., 2014). Also agro-forestry approaches with inter-planted trees can harbor soil pests, such as grubs on
Alnus tree roots [K. Li, I. Nyamwasa Institute of Plant Protection, Chinese Academy of Agricultural Sciences (IPP-CAAS), China; A. Hategekimana Rwanda Agricultural Board (RAB), Rwanda, 2014, pers. comm.]. This is in contrast to the fact that such cropping systems are usually considered beneficial, that is, they are supposed to decrease the incidence of pests and to reduce soil erosion. In addition, the use of certain monocultures can favor some of the soil dwelling species, as many of them are restricted to certain crop plants, or have long life cycles over several cropping seasons (Edwards, 1976; Li et al., 2015). Finally, it is difficult to effectively use synthetic pesticides due to problems of distribution of the active ingredients in the soil, their adsorption or rapid break down, and the impracticality of applying contact pesticides (
Furlan et al., 2006;
Mayo and Peters, 1978;
Speese, 1997). Thus pesticides often provide less control in the soil than above ground. Subsequently, soil pesticides are often highly toxic and/or concentrated. Therefore, in most regions many soil pesticides are restricted or banned due to their toxicity (Gill et al., 2012) and/or likelihood to contaminate soil or ground water (European Commission, 2009a, b). Also soil fumigants are restricted in many agricultural regions worldwide due to their high toxicity (
Anonymous, 2007; UNEP, 2007; WHO, 2009). Finally, the availability of soil pesticides can be limited (e.g. in DPR Korea; many European countries), and/or they are too expensive or impractical for smallholder growers (e.g. East Africa) (
Toepfer et al., 2014). These factors combined together present a barrier to the effective and sustainable control of soil pests. As a consequence alternative strategies are being developed and implemented. One strategy which is being actively developed globally is the use of entomopathogenic nematodes.
Entomopathogenic, also called entomoparasitic or beneficial, nematodes are biocontrol agents that are well-adapted to the soil environment and are non-toxic (Grewal et al., 2005). Entomopathogenic nematodes (EPN) can persist in soils much longer than pesticides and some can actively search for the insects (
Ehlers, 1996;
Griffin et al., 2005;
Peters et al., 1996), or for the damaged plants and subsequently their associated pests (
Rasmann et al., 2005). If appropriately selected and applied, these biocontrol agents can propagate on soil insects, and can therefore actively respond to changing pest densities; an evident advantage over pesticides (
Cabanillas et al., 2005;
Ehlers, 2003). Currently EPNs are mainly used against soil dwelling pests in high value vegetables, in greenhouses, tree nurseries, berries, seed beds of field crops or in urban grass or turf habitats as well as in mushroom production (Ehlers, 2003; Jin et al., 2003; Grewal et al., 2005). Although continuously increasing, nematode usage still lags behind the use of bacteria-based biopesticides such as
Bacillus thuringiensis or Spinosad, or botanical-based pesticides such as neem or pyrethrin.
One reason is that the establishment and local adaptation of mass production systems for EPN requires considerable development effort and time. There exist two general approaches to the mass production of EPN, in-vitro and in-vivo (Friedman, 1990; Shapiro-Ilan and Gaugler, 2002; Shapiro-Ilan et al., 2012). The in vivo production system appears to be the appropriate method for niche markets and small growers where a lack of capital, engineering expertise or infrastructure cannot justify large investments into technology. Thus, the in-vitro technology is mainly used when large scale production is needed at reasonable quality (Shapiro-Ilan et al., 2012; Shapiro-Ilan et al., 2014).
The
in-vivo mass production of nematodes is based on the use of alternative insect hosts (
Ehlers, 2001; Shapiro-Ilan and Gaugler, 2002). Such alternative hosts are usually the larvae of the mealworm [
Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae)] or the wax moth [
Galleria melonella (Linnaeus) (Lepidoptera: Pyralidae)], but other hosts are possible. Insect larvae are inoculated with infective juveniles of a nematode species/strain, the juveniles enter the insects, release their symbiotic bacteria such as
Photorhabdus or
Xenorhabdus (Kaya et al., 2006), these multiply and are the primary reason for the death of the host insect. The nematodes then feed and multiply on the bacteria inside the insect. After completing their life cycle, infective juveniles exit the insect cadavers and can then be harvested, formulated, and stored or distributed.
The
in-vitro mass production of nematodes can be based on liquid media in fermenters or on semi-solid systems using support materials such as sponges (
Ehlers, 1988;
Ehlers, 1994; Han et al., 1995; 1997; Shapiro-Ilan and Gaugler, 2002; Shapiro-Ilan et al., 2014). As for the latter approach, the symbiotic bacteria species of a specific nematode species needs to be cultured first. Then, bacteria are mass-cultured on media-soaked sponges. Once the bacteria are grown, nematodes are inoculated and incubated for mass proliferation on the bacteria and media-soaked sponge. Upon reaching the infective juvenile stage, sponge and nematodes are either stored cool, or are harvested, formulated, and distributed (
Ehlers et al., 1992). The harvested nematodes contain their symbiontic bacteria increasing their virulence on the target insects.
As the production processes are technology and knowledge intensive, the establishment of factories for the production of beneficial nematodes is difficult and requires considerable skills (
Ehlers, 2001; Shapiro-Ilan et al., 2014). Unfortunately, expertise is scattered and altogether limited in this area.
To help this process, we analyzed the design of 21 in-vitro semi-solid and/or in-vivo nematode production factories from four countries, that is, from Rwanda, Switzerland, PR China, DPR Korea. The aim was to identify optimal standard designs for mass production factories of such biological control agents and to provide insight and guidance into the process and considerations essential to planning the establishment of such factories. This knowledge is vital to the effective dissemination of such technologies to other locations in-country, or to the transfer to new countries or regions. This will ultimately lead to a more effective, more sustainable and safer control of soil insect pests, and to a reduction in early-stage crop losses, particularly in seedlings and nurseries in vegetable production, as well as in close-to-harvest crop losses, particularly in tuber crops.
Process of developing factory design criteria
The proposed designs for factories for mass production of EPNs are a result of a technical review of lessons learned from existing commercial factories in Switzerland and P.R. China, and from the recent development processes of factories in Rwanda, P.R. China, DPR Korea, totaling 21 factories in all.
These included one commercial factory at Andermatt Biocontrol AG, in Grossdietwil in Switzerland, employing the in-vitro semi-solid (media-soaked sponge) method for mass production since 1989. The other commercial factory, Lvbenyuan Biotech. Co. Ltd, is a spin-off commercial company of the Guangdong Entomological Institute, Guangdong Academy of Science, in Guangzhou in P.R. China, established in 2001. It also employs the semi-solid in-vitro method. Another in-vitro -based factory is the experimental spin-off of the Shandong Agricultural University in Taian, Shandong Province, P.R. China, established in 2012.
An experimental factory was established at the Plant Protection Institute, Academy of Agricultural Sciences, in Pyongyang in DPRK in 2012, to aid in the development of in-vitro and in-vivo mass production methods suitable for the prevailing conditions within DPRK. Subsequently, a DPRK prototype factory was established at the Mangyondae Field Station of the Central Plant Protection Station, Department of Plant Protection, Ministry of Agriculture, in Pyongyang in 2013. This factory employs both the in-vitro and in-vivo mass production methods, and served as the base for dissemination to the provincial and county levels within the DPRK agriculture sector.
Between 2013 and 2014, three in-vitro factories were established at the higher administrative provincial level of DPRK, at North Hwanghae Provincial Plant Protection Station in Sariwon City, at South Hwanghae Provincial Plant Protection Station in Haeju City, and at North Pyongan Provincial Plant Protection Station in Sinuiju City.
Similarly from 2012 to 2014, 9 in-vitro factories were established at the county level in DPRK at Anak County Plant Protection Station, Sinwon County Plant Protection Station, and at Ongjin County Plant Protection Station, all in South Hwanghae Province; Yontan County Plant Protection Station in North Hwanghae Province, Kosan County Plant Protection Station in Kangwon Province; Sukchon County Plant Protection Station in South Pyongan Province; Unjon County Plant Protection Station, Dongrim County Plant Protection Station in North Pyongan Province and at Sinchon County Plant Protection Station in South Hwanghae Province Between 2014 and 2015, one in-vitro experimental pilot factory was established at the Rubona Southern Station of the Rwanda Agricultural Board in Huye, Rwanda, developing an in-vitro method suitable for Rwanda.
In 2012, three in-vivo mass production factories were established at cooperative farms in South Hwanghae Province of DPRK, at Ryongsan Cooperative Farm in Anak County, at Woldang Cooperative Farm in Sinwon County, and at Up Cooperative Farm in Ongjin County.
During the process of factory development and establishment, as well as during local adaptations of production processes in China, DPRK and Rwanda, the successes, failures, and solutions were monitored at least every half year using internal monitoring journals as well as meeting and consultancy reports. In DPRK and Rwanda, additionally, several stakeholder meetings were held. During these meetings focus group discussions and questionnaires helped to obtain detailed information from those directly involved with the establishment of factories and implementation of the production of nematodes. In this way detailed information was gathered, giving a clear understanding of the issues encountered during the establishment of a functioning, locally adapted, mass production factory. This information provided a guide for finalizing standard mass production factory designs, influencing criteria for site selection, and internal and external factory design and room considerations for in-vitro and / or in-vivo factories.
Nematode production processes
The flow of the production process needs to be understood prior to designing a nematode mass production factory (Figures 1 to 3). There are two general types of processes, the in-vitro and in-vivo mass production methods.
In-vitro mass production process
The in-vitro production of EPNs can be based on liquid media in fermenters or semi-solid systems using carrier materials such as sponges (Ehlers, 1988, 1994; Han et al., 1995, 1997; Shapiro-Ilan and Gaugler, 2002; Shapiro-Ilan et al., 2014). Here we consider the semi-solid, sometimes called solid, system, as this is more appropriate for low-input situations, such as those encountered in less developed and developing countries, than the operationally complex liquid fermentation system.
Figure 1 shows one possible, commonly used pathway of production. The symbiotic bacteria species of a specific nematode species (Shapiro-Ilan and Gaugler, 2002; Kaya et al., 2006), needs to be cultured first. This is usually done on agar plates with subsequent inoculation of, and growth in, liquid subcultures. The liquid bacteria culture is then used to inoculate media-soaked sponge in flasks/bottles or plastic bags and incubated (Bedding, 1984; Grunder, 1985). Once the bacteria have colonized the media/sponge, nematodes are inoculated and incubated for mass propagation. Once reaching the infective juvenile stage, the flasks/bottles/bags containing sponge, bacteria and nematodes are either stored in a cool location, or are harvested, formulated, and distributed for field application (Ehlers et al., 1992).
In-vivo mass production process
The in-vivo production of EPNs is based on the use of alternative insect hosts (Ehlers, 2001; Shapiro-Ilan and Gaugler, 2002; Shapiro-Ilan et al., 2012; Shapiro-Ilan et al., 2014) (Figure 2). Such hosts are usually the larvae of the mealworm [T. molitor] or the wax moth [G. melonella]. Other larval hosts are possible, e.g. super-worm [Zophobas morio Fabricius, Coleoptera: Tenebrionidae], house fly [Musca domestica Linnaeus, Diptera: Muscidae], or silkworm [Bombyx mori (Linnaeus), Lepidoptera: Bombycidae]. Larvae are mass-reared and then inoculated with infective juveniles of a nematode species/strain. The nematodes enter the insects and release their symbiotic bacteria, which are the primary killing agents of the host insect. The nematodes then feed and multiply on the liquefied insects. After completing their life cycle and when the resources in an insect body become limited, infective juveniles exit the cadavers and can be harvested, formulated, and stored or distributed for field application.
Factory design criteria
Criteria for establishing mass production factories for EPNs are cross-cutting but can be grouped into three broad areas: (i) Criteria for factory site selection; (ii) Criteria for external and internal factory design; (iii) Criteria for room design.
Criteria for factory site selection
Access to agricultural sites: Nematodes are sensitive to high temperatures and need careful handling to prevent mortality during transport to farm or field locations for application. Where infrastructure and transport are limited, it is recommended to locate a factory, regardless of the production method, within close proximity to farming areas.
Access to electricity: Access to a reliable supply of electricity is necessary for key activities within the production process, particularly for the in-vitro production system. Electricity should ideally be available continuously for a 24 h period every day, or with no more than 1 to 2 h power cuts. At a minimum, 3 to 5 h of electricity are needed each day at critical stages in the in-vitro production method and 1 to 3 hours for the in-vivo method.
Electricity is ideally required for air conditioning units (heating and/or cooling of rooms), incubators, fridges, laminar flow bench (only for in-vitro method), media preparation, autoclaving, and light. In addition, electricity is needed for a 2 × 24 h period for the liquid culture of the symbiotic bacteria on a shaker or magnetic stirrer once every week, or every other week, depending on the production cycles (in-vitro only). Alternatively, battery- or petrol-based generators may be used. Some autoclaves need a high voltage connection.
Access to water: There should be a water supply, such as electric- or hand-pumped from a well or a storage tank and/or a gravity fed water source from a local reservoir or tank. This is to prepare culture media and to harvest nematodes, as well as for cleaning of tools.
Building to contain factory: The location should have a pre-existing building which is able to house the production factory in its entirety. Alternatively, a suitable site should be available to enable construction of a new building. The building should, regardless of the production method, consist of at least six and up to 11 rooms (Figures 4 and 5). The building area for the in-vitro mass production system should be 120 to 180 m2, and for the in-vivo system 140 to 200 m2. Those sizes are little related to the production output rate, but related to ease of working.
Access to qualified staff: Skilled personnel consisting at least of one, better two, technical production personnel familiar with/ or trained in laboratory work as well as of one at least half-time manager (production engineer) should be available to operate the factory. In addition, an entomologist or agricultural extension staff would help to advice on nematode application.
Criteria for external and internal factory design
In considering external and internal factory design, it is important to understand the production process and workflow (Figures 1 to 3), required rooms (Figures 4 and 5) and conditions required for insect, bacteria and nematode production and storage (Table 1).
The room allocation in a factory should follow the production flow; ideally all rooms should be in the same building. Consideration should be given to room separation and distances for minimizing cross contamination between cultures, having culture rooms in well-insulated areas and near to the heating source, and having the cool room at the coolest part of a building, preferably below ground. Energy efficiency and existing structures within a building need to be considered.
In many ways the construction of a new building enables better design to overcome possible issues with temperature control, access to water, and room allocation to maximize work flow. For room sizes refer to section “room design” below and in Table 1.
In addition, other criteria should be addressed for both new and pre-existing buildings as follows:
Orientation of building and insulation: The building should be designed so that the cool room, and in warm regions also the bacteria and nematode culture rooms are not facing the sun. This will reduce the amount of insulation and electricity needed for air conditioners. An internal corridor on the sun-exposed building side can be used to facilitate this (Figures 5 and 7). Nevertheless, at least the outer facility walls need to be insulated, such as through the construction of double walls internally, or other insulation systems. Similarly, the roof cavity should be well insulated, and in some cases the insertion of a second ceiling is needed.
Alternatively, the factory could be placed in a cellar or on the ground floor of a multiple storey building (Figure 6); or a basement situation may be created by piling up soil 1.5 to 2 m around the building. In some cases, the entire factory may be constructed below-ground.
Cool room placement: Bacteria and nematode storage need cool conditions. Therefore one of the rooms of the building should be a cool cellar or must allow the installation of a cool cellar (Figures 6 and 7). Buildings in areas with high ground water level are to be avoided. In regions with low electricity costs, a cellar could be replaced by a well-insulated room that is cooled through powerful cooling systems.
Media preparation and harvesting room(s) placement: These rooms need to be placed close to the water source and a good drainage system, and require space and lots of light.
Autoclave room placement: This room needs to be placed close to a good power source, and often requires a high voltage connection. The room must also have an outer wall, as ventilation systems need to be installed because of the steam. If the electricity supply is insufficient a coal or wood powered steam oven needs to be built, in a suitable place attached to the building.
Clean (sterile working) room placement: This room needs to be placed near the media preparation and bacteria culture rooms to keep workflow short and to avoid contamination (see for room details below). If light for electricity is available, this room should preferably be placed inside a building, as opposed to against an outer wall.
Bacteria and nematode culture room(s) placement: These rooms need to be placed in well-insulated areas of a building and away from insect rearing to avoid cross contamination. Proximity to the media preparation room and to the cool room would be advantageous. Room details are given thus.
Insect rearing room(s) placement: These rooms are usually the largest rooms of a factory. They need to be ideally placed distant from the sterile room, and from bacteria or nematode cultures. For some insects, such as Tenebrio sp., ventilation is needed, and therefore insect rearing room (s) are usually along the outer wall of a building. In regions with cold winters, however, such rooms are often placed in well-insulated areas of a building.
Following the above criteria as a guide, compromises can be made in finding the optimal solution for a factory building design and room allocation that fits best to the local situation. Suggestions are made in Figure 3 for the in-vitro based factories and in Figure 4 for the in-vivo based factories. Figures 5 to 7 give real examples.
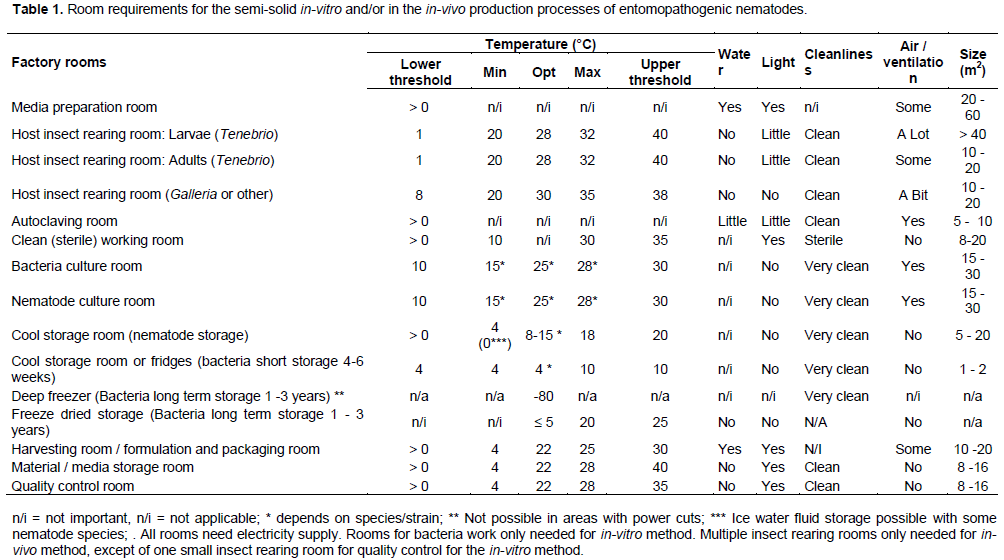
Criteria for room designs
Media preparation room: The function of this room is to prepare media for (a) alternative insect host rearing for nematode in-vivo culture, (b) for bacteria cultures (agar, liquid, semi-solid on sponge), (c) for nematode cultures (semi-solid on sponge) and (d) for cleaning of tools.
Media preparation includes weighing, heating/boiling, diluting, mixing, and sterilization. The room needs to have water access, drainage, electricity, and in regions with cold winters also heating. In countries with limited electricity, large windows are needed to have enough light for working.
The room is usually about 40 to 60 m2, but at least 20 m2. The room may also accommodate activities which could be allocated in their own rooms, dependent on the factory building size. These include, autoclaving room, harvesting room, formulation and packaging room, quality control (e.g. bioassays), material/media storage room, and/or office.
Host insect rearing room (Larvae): The function of this room is to rear insect larvae that are alternative hosts of nematodes. Large scale rearing is needed to allow the in-vivo culture of nematodes. Small scale rearing is needed for nematode strain maintenance (stock culture) and for bioassays (quality control). Typical hosts are the larvae of T. molitor or G. melonella, but other hosts are possible. Most insect larvae need warm but not hot conditions for mass rearing. In regions with cold winters, heating systems are needed in the rearing rooms.
All Galleria life stages can be reared in one room under dark conditions, or even in a set of incubators. Tenebrio rearing ideally needs two rooms, a small one for adult and egg rearing (see below), and a large one for larvae and pupae rearing. Although not optimal, this can also be housed in one room. Tenebrio larvae are reared in large well-ventilated rooms with space for many shelves for flat open rearing trays. Such rooms are usually of more than 40 m2.
Insect rearing rooms must always be well-separated from nematode culture rooms as well as from harvesting and formulation room, and should have no direct door connection between them.
Host insect rearing room (Adults): The function of this room is to rear the adults of the alternative insect host of nematodes and to produce eggs that allow larvae production for in-vivo nematode culture, for nematode strain maintenance (stock culture), and for bioassays (quality control). For some host insects, such as Tenebrio, adult and egg rearing needs, ideally, to be separated from larvae and pupae rearing (see above). Tenebrio adults need dark conditions, or only little light. Such rooms are usually about 10 to 20 m2. The room must always be well-separated from nematode culture rooms and should have no direct door connection between them.
Autoclaving room: The function of this room is to house the autoclave for sterilizing media for the bacteria and nematode culture, that is, large amounts of flasks/bottles or plastic bags with media soaked sponge. Also working tools need to be sterilized. Next to an autoclave, the room usually also contains a microwave for quick sterilization. The room needs to have good electricity supply, and for some autoclaves high voltage (380V) access. The room needs to have a ventilator so that steam can exit the room. The room is often combined with the media preparation room. The room is usually only about 5 to 10 m2.
Clean (Sterile working) room: The function of this room is to allow (a) sterile inoculation of media plates and liquid
media flasks with bacteria for their sub-culturing and scale-up, (b) sterile inoculation of media-soaked sponge with bacteria in flasks/bottles/plastic bags, (c) inoculation of bacteria-containing sponge media in flasks/ bottles/ plastic bags with nematodes (Figure 1). In addition, the occasionally required isolation of bacteria from infected insect hosts is done under sterile conditions. The room ideally has a place for a laminar flow cabinet and electricity access, although it is possible to work aseptically without a flow cabinet using a cleaned table and a flame (ethanol burner, candle, etc.). No windows are needed, except in countries with limited electricity, where windows would provide light for working. Any window must remain closed to allow clean conditions and doors need to be well-sealed. Double doors are an advantage.
The room is usually about 8 to 20 m2. The room should not be combined with other rooms to ensure sterile working conditions. This room is not needed for in-vivo nematode production factories.
Bacteria culture room: The function of this room is to (a) scale-up bacteria sub-cultures, i.e. liquid media bacteria flasks on shakers, and (b) for mass culture of bacteria on media-soaked sponge in flasks/bottles/bags. This room is not needed for in-vivo nematode production factories.
The room needs good temperature control that is, heating in cold regions/seasons, cooling in warm regions/seasons (Table 1). Effective room insulation is needed. Therefore, culture rooms are often placed below ground or in the center of buildings. As mass culturing of bacteria produces heat, often an additional cooling and/or ventilation is needed. Bacteria culture rooms are kept dark and do not need windows. Shakers need constant power supply.
The room is usually about 15 to 30 m2. The room should not be combined with other rooms to avoid cross contaminations and to assure temperature appropriate for culturing different bacteria. In cases where bacteria and nematode culture need similar conditions, and where space is limited, both cultures may be combined in one room.
Nematode culture room: The function of this room is to mass culture nematodes on bacteria and media- soaked sponge in flasks/bottles/bags. The room needs temperature control that is heating in cold regions/seasons, cooling in warm regions/seasons. Effective room insulation is needed. Thus, culture rooms are often placed below ground or in the center of buildings. As the mass culture of nematodes produces heat, often an additional cooling and/or ventilation is needed. Nematode culture rooms are kept dark and do not need windows.
The room is usually about 15 to 30 m2. The room should not be combined with other rooms to avoid cross contaminations and to assure temperature appropriate for specific nematodes. In cases where bacteria and nematode culture need similar conditions, and where space is limited, both cultures may be combined in one room.
Cool storage room: The function of this room is to store nematodes prior to their use. Nematodes deplete their nutrient sources when being active, that is, in warm conditions, and when not within an insect host. Therefore, nematodes are usually stored on sponge in the culture flasks/bottles/bags. Sometimes they are stored in flat well-aerated water bottles/flasks. Depending on the nematodes species/strain they can be stored for 1 to 3 months. They need to be stored at constantly cool temperature of between 5 to 15oC depending on the nematode species/strain (Table 1).
In countries with limited access to electricity for cooling systems, cool rooms are usually placed below ground and on the opposite side of the building from the sun (external / internal factory design are given previously in the study). Some countries add ice blocks to the below ground rooms. Some countries do not work with cool rooms but with a number of large fridges.
The size of the room needs to be as small as possible to allow cooling but big enough to allow storage of all culture flasks/bottles/bags and products. Thus the room is usually between 5 and 20 m2. The room cannot be combined with other rooms.
Harvesting room: The function of this room is to enable harvesting of the infective juveniles of the mass-reared nematodes from culture flasks/bottles/bags. This is done manually in water trays, or by using washing machines. Subsequently, nematodes are concentrated through sedimentation in tanks or trays, and/or through filtering systems. Sedimentation tanks need space. Water access and good drainage systems are required. The room is usually between 10 and 20 m2. The room is sometimes combined with the media preparation room and/or with the formulation and packaging room.
Formulation and packaging room: The function of this room is to formulate the nematodes for transport to the users and to ease application. The room does not have any special requirements.
The room is usually about 10 to 20 m2. The room is often combined with the media preparation room and sometimes with the harvesting room.
Quality control room: The function of this room is to monitor the quality of bacteria and nematodes throughout all production steps. The room usually contains stereomicroscope(s) and space for small soil/sand containers with insect larvae for bioassays. In countries with limited electricity, windows are needed to allow sufficient light to work. The room is usually about 8 to 16 m2. The room is sometimes combined with the media preparation room or with the office.
Material/media storage room: The function of this room is to store media and materials for the insect rearing and/or for the bacteria and nematode culture. No special requirements are needed for this room. The room is sometimes combined with the media preparation room. The room is usually about 5 to 10 m2.
Office: The function of this room is to organize all records from the factory. The room is usually about 5 to 20 m2. The room is sometimes combined with the media preparation room or with the quality control room.
Training room: This room is optional, and used for product presentations and for training technical personnel in the mass production of nematodes, and users in nematode product handling and application. The room is usually between 20 and 40 m2. The room is sometimes combined with the office room.
Reviewing a large array of nematode-based biological control agent production factories, this paper succeeded in concluding maximum, minimum and optimal factory designs for two different production methods for EPNs (Figures 4 and 5). These findings are based on the majority of such factories currently known to us to exist globally. Experiences and lessons learnt from over 15 years are summarized. We therefore believe to propose suggestions and solutions that are valuable for others aiming at producing EPNs, regardless of their situation and location. Thus, we hope that this can ultimately lead to a decrease in the reliance on synthetic pesticides in the control of soil insect pests, and/or provide a useful tool to control soil insect pests where none is currently available.
Unfortunately, factory designs are rarely published due to concerns over intellectual property and maintaining competitive advantage. Moreover, to our knowledge, not many other nematode mass productions factories exist across the world that are based on the in-vivo or semi-solid in-vitro production method as discussed here. There exists at least one additional factory in Pakistan that also followed the in-vitro production approach described here, although lessons learnt are not available (National Nematode Research Centre (NNRC), University of Karachi Pakistan).
As a consequence of the above-mentioned restrictions, we are limited in comparing our findings to other publications. However, we show some examples in Figures 6 and 7 how the proposed standard designs were realized, making compromises according to local conditions. It can be seen that each factory owner tried to follow the suggestions presented here, but had to find compromises due to the restrictions of the existing building structures. Many facilities were struggling in achieving good insulation. For the in-vitro mass production system 120 to 180 m2 were usually needed, for the in-vivo system 140 to 200 m2. Interestingly, factory and room sizes were little related to the production output rates, but rather related to ease of working. Most factories were established over a period of six months to one year. Subsequent staff training took another six months to one year until the staff was fully enabled to realize continuous and successful mass production cycles. It can therefore be considered that it takes overall one to two years until a factory is mass producing the EPNs suitable for biological control of insect pests.
Currently, 17 of the 21 factories included in this study produce large quantities of nematodes for every cropping season. This is an impressive success rate and deserves recognition. The majority of nematode products from these factories in East Asia are currently applied against grubs, wireworms and cutworms in young vegetable plantings and onto seed beds such as of maize to prevent pest damage just after transplanting (Kang, S.I., 2012, Academy of Agricultural Sciences, DPRK, pers. comm.); or against grubs in peanuts, and chive maggots in Allium crops (Pan and Xia 1993; Liu et al., 2007). The factory in East Africa (Rwanda) is intended to produce nematodes against grubs and weevils in tuber crops, particularly in agro-forestry areas, against bean flies in bean crops, or cutworms in young vegetable plantations (Hategekimana A., RAB, 2014, pers. comm.). In the other biological control key regions of the world, that is, South America and Europe, EPNs are currently mainly used against soil dwelling pests in high value vegetables, in greenhouses, tree nurseries, mushrooms, berries, and in urban grass-habitats (Ehlers, 2003; Grewal et al., 2005).
Interestingly, the semi-solid system of nematode mass production seems to be, in the cases presented here, the preferred option over the in-vivo system. This is a result of problems with contamination in the in-vivo process, its relatively limited output, and the labor and time investment required for insect rearing. The in-vivo production system, as in line with the analysis of Shapiro-Ilan et al. (2013), appears to be the appropriate method for niche markets and in areas with small growers, where a lack of capital, engineering expertise or infrastructure cannot justify large investments into the in-vitro culture technology. The three in-vivo factories, analyzed here, are run by two to four staff, including technician(s) , a part-time factory manager (production engineer), and sometimes a part-time accountant and/or research and development staff. The amount of staff is about the same for the semi-solid in-vitro technology. Nevertheless, this technology is mainly used when larger scale production is needed at reasonable quality and in-put/labor cost. However, even the output of this technology is somewhat limited, so that a number of companies switched to the knowledge and technology intensive liquid media production system using fermenters (Ehlers, 1988; Ehlers, 1994). However, ultimately, the local situation determines the technology to be used, as each approach has its own advantages and disadvantages relative to production cost, in-put costs and input availability, technical know-how required, economy of scale, and product quality; and each approach has the potential to be improved (Shapiro-Ilan and Gaugler, 2002; Shapiro-Ilan et al., 2012). Finally, it remains to be mentioned that the design of a nematode mass production factory is only one factor in success. When an EPN is used against a pest insect, it is critical to match the right nematode species/strain against the target pest (Shapiro-Ilan et al., 2012). Moreover, biotic factors including nematode pathogens, predators and other soil organisms, as well as abiotic factors such as ultraviolet radiation, soil moisture/relative humidity, temperature, or timing can affect nematode application efficacy. Additional research towards lowering product costs, increasing product availability, enhancing ease-of-use, and improving efficacy and carryover effect (field persistence) will stimulate further use of nematodes in biological control.
It is hoped that the proposed standard designs for mass production factories of such biological control agents can provide insight and guidance into the process and considerations essential to planning the establishment of such factories. This knowledge will be vital to the effective dissemination of such technologies to other locations in-country, or to the transfer to new countries or regions. It will help growers in regions, where pesticides are not accessible or too toxic. This will also benefit the implementation of alternative agricultural production systems. Ultimately, consumers across the world will profit from safer food.