ABSTRACT
The purpose of treating seeds chemically is to eradicate their pathogens and/or protect them against soil pathogens, mainly by germination time. However, there is little research on vegetables investigating the effect of this treatment on seed quality. Therefore, this study evaluates the effects of Carboxin + Thiram doses on germination and vigor of three lots of broccoli seeds, as well as on the incidence of fungi in treated seed. The 15 treatments were evaluated in a factorial system (3x5), with the first factor consisting of three lots of 'Avenger' broccoli seeds (lots 82744, 82745 and 82749), and the second factor consisting of five doses (0, 0.04, 0.06, 0.10 and 0.12% of a.i.) of Carboxin + Thiram fungicide (commercial name Vitavax-Thiran). The germination and seed vigor were evaluated, in addition to the presence of pathogens in seeds after treatment (blotter test). All lots showed high levels of germination and vigor. The lot 82749, however, showed higher value in plug test in substrate emergence (99%) than lot 82745 (95%). Regarding the treatment with Carboxin + Thiram, no changes in germination average (98%) and vigor were noticed (average for the first germination count, length, and dry weight of seedling, plug test at 10 days after sowing of 97%, 4.9 cm, 4.0 mg and 96%, respectively), showing that this fungicide, in the evaluated doses, does not affect the quality of broccoli seeds. As to seeds health, the pathogens Alternaria spp. and Fusarium spp. were detected, in addition to saprophytic species such as Penicillium, Aspergillus, Trichoderma, and Rhizopus. The higher incidence of Fusarium spp. was noticed in lot 82744, and the lowest in lot 82749. As to Penicillium spp., lot 82479 was the most contaminated. Regarding other fungi, the general incidence was very low and there was no difference between lots and doses used.
Key words: Brassica oleracea, seed treatment, fungicides.
It is the seed physiological and seed sanitary quality that will determine their performance in the field, that is, the proper establishment of plants, which is essential for satisfactory levels of productivity and final product quality (Nascimento et al., 2011). It is not always possible to obtain seed lots with 100% guaranteed disinfection of pathogens. Also, it is not possible to ensure that the soil or substrate will be free of pathogens. Hence, in most cases, treating seeds of vegetables, particularly those whose seeds are of highest value, is required. The purpose of treating seeds chemically is to eradicate their pathogens and/or protect them against soil pathogens, mainly by germination period. Furthermore, as small quantities of products per unit area are used, there is less risk of environmental pollution (Carvalho and Nakagawa, 2000).
According to Menten and Moraes (2010), there were 19 active fungicide ingredients registered for seed treatment in Brazil, although these registration covers some species only. Among those ingredients, one of the most used is the Carboxin + Thiram because, according to Marini et al. (2011), it provides greater protection to seeds against pathogens found in soil and in the seed itself, especially when exposed to unfavorable development conditions.
There are studies assessing the effects of this fungicide on seed physiological quality of cotton (Faria et al., 2003), castor plant (Tropaldi et al., 2010; Santos et al., 2012), peanuts (Bittencourt et al., 2007), rice (Schuch et al., 2006; Lobo, 2008; Morales et al., 2012), maize (Fessel et al., 2003), safflower (Rogério et al., 2012), and wheat (Marini et al., 2011), among other species. However, no research on vegetables was found that studied the fungicide effect on seed quality. The vast majority of vegetable seeds sold in Brazil are treated with fungicides, as their quality increases and the treatment cost is low, mainly for hybrid seeds, whose price is high.
The seed treatment effectiveness depends, among other factors, on the seed species and vigor, which may vary from lot to lot (Menten and Moraes, 2010), and the treatment should not affect the seeds physiological quality. According to Cardoso and Silva (2009), seeds of high physiological quality are essential to brassica production components, as they favor strong, uniform, and healthy seedlings. Therefore, this study evaluates the effects of Carboxin + Thiram doses on germination and vigor of three lots of broccoli seeds, as well as on the incidence of fungi in treated seed.
The experiment was conducted in the Vegetable Seeds Laboratories of the Horticulture and Plant Protection Departments, of the Universidade Estadual Paulista (UNESP), Botucatu City, São Paulo State, Brazil. The 15 treatments were evaluated from a 3x5 factorial system, with the first factor consisting of three lots of Sakata® 'Avenger' hybrid broccoli seeds (lots 82744, 82745 and 82749), and the second factor consisting of five doses (0, 0.04, 0.06, 0.10 and 0.12% of a.i.) of Carboxin + Thiram.
The commercial product used was VitavaxThiram®, which contain the following active ingredients (a.i.): 5,6-dihydro-2-methyl-1,4-oxathi-ine-3-carboxanilide (Carboxin, 200 g L-1, that is, 20% w/v), and Tetramethylthiuramdisulfide (Thiram 200 g L-1, that is, 20% w/v), and Ethylene Glycol (249 g L-1, that is, 24.9% w/v), and other ingredients (507 g L-1, that is, 50.7% w/v). It is a systemic and contact fungicide of the Carboxanilide (Carboxin) and Dimethyldithiocarbamate (Thiram) chemical group, and used in seed treatment. The evaluated doses correspond to the following commercial product (c.p.) doses: 0, 0.2, 0.3, 0.5 and 0.6% of c.p.
The application was done in rotating pans, with a central disk inside and in the middle, also rotating, but in the opposite direction, for product distribution purposes. The device is known as “Rotary” (Seed Processing Holland®). After being treated and dried, the seed physiological and seed pathological issue were evaluated according to the following tests:
(i) Germination: Standard Germination Test (SGT) according to the Seeds Analysis Rules (ISTA, 2004; Brasil, 2009). Gerboxes were used, with two sheets of moistened germitest paper with 2.5 times their weight of distilate water, and four replicates (boxes) of 50 seeds, totaling 200 seeds per treatment. The boxes were placed in germination chamber at 20°C. The count of normal seedlings done on day 10 after sowing (DAS 10), with the value expressed in percentage;
ii) Germination Test First Count (GFC): the normal seedlings were counted on DAS 5, based on the SGT, with the value expressed in percentage;
iii) Length of shoot: in a random sample of ten seedlings evaluated on DAS 10 in SGT, the seedlings shoot length was measured with a ruler, and the value was expressed in cm;
(iv) Seedling dry matter weight: all normal seedlings evaluated on DAS 10 in SGT were placed in an oven with forced air circulation, at a temperature of 40°C, with subsequent weighting of total dry mass using analytical scale (0.1 mg accuracy ). After dividing the value found by the number of seedlings, the amount of dry matter per plant was calculated in milligrams;
(v) Plug test: polypropylene trays were used to produce vegetable seedlings, containing 162 cells (31 cm3 per cell) with Tropstrato® substrate, a pathogen free substrate, kept in a greenhouse during the assessment. Four replicates of 50 seeds per treatment were used. The emergence assessment was made on DAS 5 and DAS 10. Seedlings were considered emerged when cotyledons were fully open.
For the purposes of these seed physiological quality analyses, the experiments were fully random, with four replications always.
(vi) Seed pathology analyses: It was employed the blotter test, which consisted in distributing 25 seeds over three sheets of moistened filter paper previously prepared on petri dishes. Eight replications, totaling 200 seeds per treatment, were used. The plates were kept at 20 ± 2°C for a twelve-hour photoperiod under white fluorescent light during seven days. The seeds were evaluated individually under a magnifier, with the results expressed in percentage of seeds with fungus.
For seed pathology analyses, the results achieved for each fungus were processed separately in arc sin Ö(x/100) to have the statistical analysis performed. The data obtained for all traits were subjected to variance analysis and the averages were compared by Tukey test at 5% probability.
The seeds physiological quality test results may be found in Table 1. There was no interaction between the factors (fungicide lots and doses) in all variables considered, indicating independence between them. Regarding lots, no differences in total germination was found, with a 98% average (Table 1), that is much higher than the minimum standard allowed for marketing in Brazil by the Ministry of Agriculture, Livestock and Supply (MAPA), which is 75%. Also, no differences were found for the standard germination first count, with a 97% average. The first count (DAS 5) of seeds is considered as vigor test, in which samples that germinate faster, with higher percentage of normal seedlings on that date, are considered to be the strongest (Marcos Filho, 2005; Baalbaki et al., 2009). The 97% average achieved demonstrates the high seed vigor of all lots.
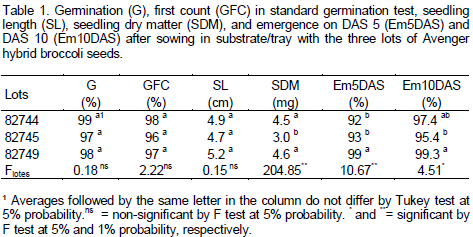
Despite there was no difference in seedling length on DAS 10 in SGT, with an average of 4.9 cm (Table 1), a lower seedling dry weight was observed in this same assessment (DAS 10) on lot 82745. This smaller vigor of this lot was confirmed in the emergence test, both on DAS 5 and DAS 10. Lot 82749 showed higher emergence in substrate on DAS 5 than the other two lots. Despite the small differences observed, with the highest vigor for lot 82749 and the lowest for lot 82745, the emergence test figures achieved were high for all lots, with a minimum of 92% on DAS 5 and 95% on DAS 10.
Some authors relate seed vigor to seedling production. Franzin et al. (2005) concluded that seed lots with higher initial quality, as detected by laboratory germination and vigor tests, produced seedlings with greater weight. The same was observed by Rodo and Marcos Filho (2003) in onion. However, in this study, the laboratory tests (germination and first count) were less sensitive than the "field" test, with production of seedlings in trays under uncontrolled temperature conditions. As to Carboxin + Thiram doses, there were no differences for all traits (Table 2), both by analysis of variance (F test) and by Tukey test, and both at 5% probability, which demonstrates that fungicide did not affect the seed quality, regardless of lot. Bittencourt et al. (2007) found no phytotoxic effect of this fungicide on peanut seed, and observed a greater emergence of treated seeds against the untreated control, because the fungicides reduced the incidence of "damping-off" caused by fungi in seeds and in the soil. Similar results were reported by Arsego et al. (2006) and Lobo (2008) with rice, and by Tropaldi et al. (2010) and Santos et al. (2012) with castor seed treated with this fungicide. Despite no phytotoxicity has been observed during treatment with the fungicide, the treated seeds in this study, regardless of dose, did not differ from untreated control for both in germination and emergence in plug test, probably because (a) it was observed that the presence of pathogens in seeds was low in all treatments, including the control (Tables 3 and 4), (b) a pathogen-free commercial substrate was used in the plug test, and (c) seed treatment with this fungicide does not interfere in the germination and vigor. Pinto (1998) found no difference in the emergence of treated and untreated sorghum seeds in sterilized soil, but noted higher emergence in seeds treated in unsterilized soil. Unlike the "large crops", it is a routine in technified systems for most vegetables, including broccoli, to have their seedling production in specific trays with fungi-free substrates (Minami, 2010).
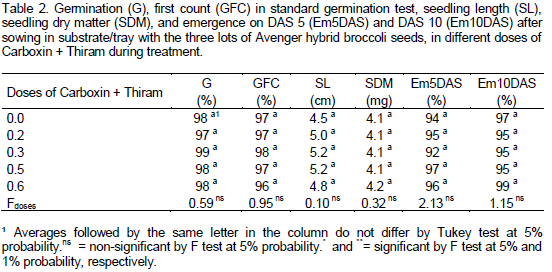
Working with wheat crop, Marini et al. (2011) reported a reduction in germination and vigor of seeds treated with Carboxin+Thiram. Faria et al. (2003) observed that although there was an increase in germination and emergence of cotton seeds treated with Carboxin + Thiram, the seedlings were smaller and with less dry matter. Morales et al. (2012) observed higher vigor of rice seeds treated with this fungicide after seed storage for 60 and 90 days, compared to control. In all the works mentioned, the doses used were within the recommended range for each species, showing the importance of the study of different species, as results may be not equal. Furthermore, the sensitivity to fungicide treatment may vary, depending on the initial seed vigor (Lobo, 2008; Menten and Moraes, 2010). In this work, despite the difference in vigor between lots, the lots were not affected by treatment with fungicide, regardless of dose. Furthermore, as small quantities of fungicide per unit area are used, there is less risk of environmental pollution compared to foliar application.
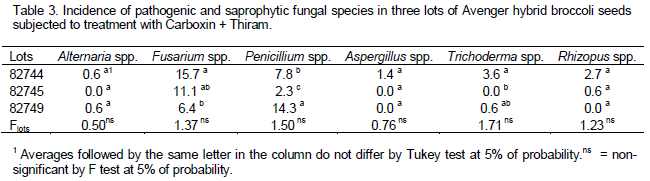
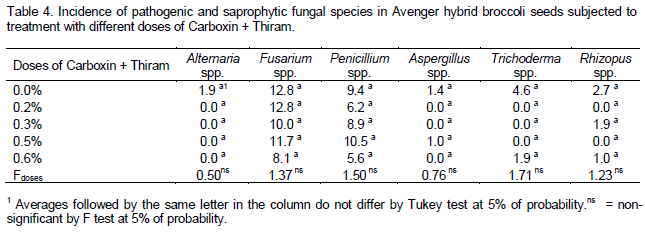
As to seeds pathology (Tables 3 and 4), the pathogens Alternaria spp. and Fusarium spp. were detected, in addition to saprophytic species like Penicillium, Aspergillus, Trichoderma and Rhizopus. The higher incidence of Fusarium spp. was noticed in lot 82744, and the lowest in lot 82749. The incidence of Alternaria spp. was low, with no difference between lots and between doses; this fungus was only detected in seeds that were not treated with the fungicide, showing the effectiveness of treatment. Among the saprophytic fungi, as to Penicillium spp., all lots differed, being lot 82479 (Table 3) the most contaminated. However, there was no significant difference between the doses tested (Table 4). As to the other fungi, the general incidence was low and there was no difference between lots and doses used.
The author(s) have not declared any conflict of interests.
It is concluded that lot 82749 showed greater vigor than lot 82745, and the seed treatment with Carboxin + Thiram in the evaluated doses did not affect the physiological quality of seeds, so all doses tested (0 to 0.12% of p.a.) can be used. The incidence of fungi was very low in all lots, showing their good seed pathology quality.
REFERENCES
Arsego O, Baudet L, Amaral AS, Hölbig L, Peske F (2006). Coating rice seeds with synthetic solution of giberellic acid, fungicides and
polymer. Rev. Bras. Sementes 28:201-206. CrossRef
Baalbaki R, Elias S, Marcos-Filho J, McDonald MB (2009). Seed vigor testing handbook. Association of Official Seed Analysts, Ithaca 341p.
Bittencourt SEM, Mentem JOM, Araki CAS, Moraes MHD, Rugai AD, Dieguez MJ, Vieira RD (2007). Eficiency of the fungicide carboxin + thiram in peanut seed treatment. Rev. Bras. Sementes 29:214-222. CrossRef
Brasil (2009). Ministério da Agricultura, Pecuária e Abastecimento. Regras para análise de sementes. Mapa/ACS, Brasília P. 399.
Cardoso AII, Silva N (2009). Influence of cultivar and seed size on cauliflower production. Rev. Ceres 56:777-782. CrossRef
Carvalho NM, Nakagawa J (2000). Sementes: ciência, tecnologia e produção. 4. ed. FUNEP, Jaboticabal, Brasil P. 588.
Faria AYK, Albuquerque MCF, Cassetari Neto D (2003). Physiological quality of cotton seeds submitted to chemical and biological treatments. Rev. Bras. Sement. 25:121-127.
CrossRef
Fessel AS, Mendonça EAF, Carvalho RV, Vieira RD (2003). Effect of chemical treatment on corn seeds conservation during storage. Rev. Bras. Sementes 25:25-28. http://www.scielo.br/pdf/rbs/v25n1/19626.pdf
Franzin SM, Menezes NL, Garcia DC, Santos OS (2005). Effect of seed quality on lettuce seedlings development. Horticult. Bras. 23:193-197. CrossRef
ISTA - International Seed Testing Association (2004) International rules for seed testing. ISTA, Zürich: P. 206.
Lobo VLS (2008). Effects of chemical treatment of rice seeds on leaf blast control and physiological and sanitary quality of treated seeds. Trop. Plant Pathol. 33:162-166. CrossRef
Marcos Filho J (2005). Fisiologia de sementes de plantas cultivadas. Fealq Piracicaba Brasil P. 495.
Marini N, Tunes LM, Silva JI, Moraes DM, Olivo F, Cantos AA (2011). Carboxim Tiram fungicide effect in wheat (Triticum aestivum L.) seeds physiological quality. Rev. Bras. Ciênc. Agrárias 6:17-22. CrossRef
Minami K (2010) Produção de mudas de alta qualidade. Degaspari, Piracicaba, Brasil P. 440.
Menten JO, Moraes MHD (2010). Seeds treatments: history, types, characteristics and benefits. Inform. Abrates 20:52-53. CrossRef
Morales M, Moratinos H, Gonzales T, Madriz P (2012). Effect of fungicides on physiology and health of rice seeds during storage. Rev. Faculdad Agron. Univ. Zulia 29:505-524. http://revfacagronluz.org.ve/PDF/octubre_diciembre2012/v29n4a2012505524.pdf
Nascimento WM, Dias DCFS, Silva PP (2011). Qualidade da semente e estabelecimento de plantas de hortaliças no campo. In.: Nascimento WM. Hortaliças: tecnologia de produção de sementes. Embrapa Hortaliças Bras. pp. 79-106.
Pinto NFJA (1998). Treatment of sorghum seeds to control of fungi in soil and associated to seeds. Summa Phytopathol. 24:26-29.
Rodo AB, Marcos Filho J (2003). Onion seed vigor in relation to plant growth and yield. Horticult. Bras. 21:220-226.
Rogério F, Silva TRB, Santos JI, Migliavacca RA, Cazado JF, Arieira CRD, Salvestro AC, Oliveira VB, Lima WS (2012) Seed treatment influence with carboxin + thiram to initial development of safflower plants. J. Food Agric. Environ. 10:675-676. CrossRef
Santos JI, Silva TRB, Rogério F, Oliveira VB, Migliavacca RA, Felix JC (2012). Seed treatment influence with carboxin+thiram to initial development of castor plant. J. Food Agric. Environ. 10:443-444. CrossRef
Schuch J, Lucca Filho O, Peske ST, Dutra L, Brancão M, Rosenthal M (2006). Physiological and sanitary quality of rice seeds stored with differents seed moisture contents and fungicide treated. Rev. Bras. Sementes 28:45-53. CrossRef
Tropaldi L, Camargo JA, Smarsi RC, Kulczynski SM, Mendonça CG, Barbosa MMM (2010). Physiological and health quality of castor seeds submitted to different chemical treatments. Pesquisa Agropec. Trop. 40:89-95.