Full Length Research Paper
ABSTRACT
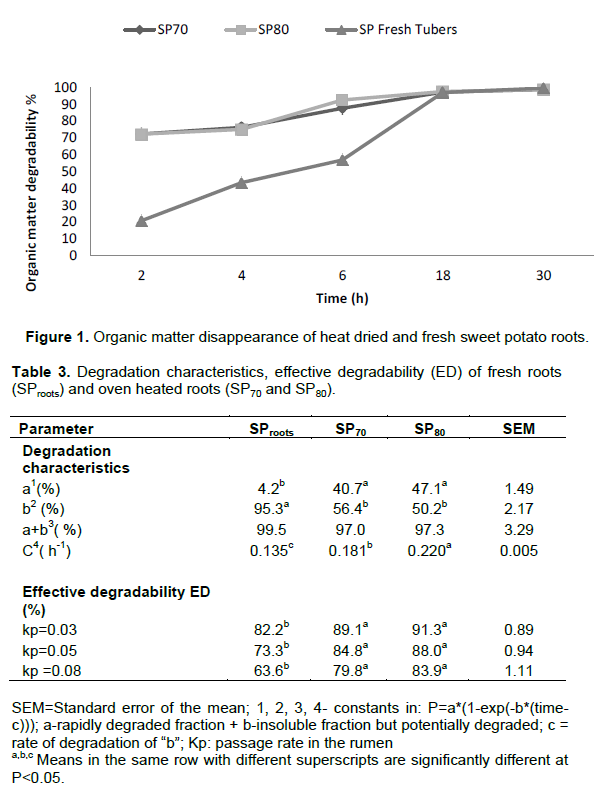
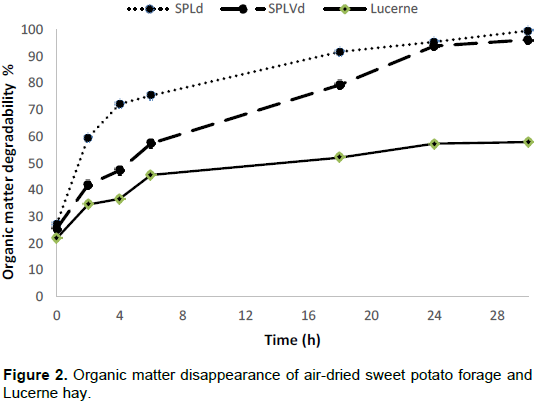
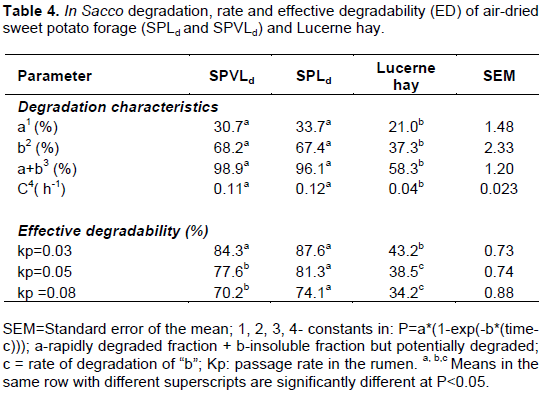
Post-harvest management of sweet potatoes (SP) crop residues preserves nutrients, deactivates inhibitor compounds and improves rumen degradation. The aim of the study was to determine effects of drying crop residues and heating roots on forage value of a bio-fortified orange fleshed sweet potato (OFSP) variety in South Africa. The crop was harvested at maturity and roots separated from crop residues. Roots were washed, sliced and divided into three portions as SProots that were frozen at -4°C for 4 weeks, SP70- oven dried at 70ºC for 8 days and SP80 -80ºC for 7 days. Aboveground crop residue were separated into portions of vines and leaves (SPVLf) and leaves and petioles only (SPLf). A subsample of each portion was air dried for 7 days (SPVLd and SPLd, respectively). Chemical composition and in sacco organic matter disappearance were determined. Crude protein (CP) was higher (P<0.05) in SPLd (24.9% CP DM) compared to fresh material with 6.5%. Neutral detergent fibre (NDF) and insoluble CP (NDFICP) were higher after drying, non-fibre carbohydrates (NFC) declined and vitamin A declined. Effective degradability (ED) was higher than for Lucerne hay and differed between SPVLd and SPLd 77.6% and 81.3% at kp=0.05; respectively. The SProots were low in CP, ether extracts and fibre; had higher NFC (77% DM) and gross energy (4.1 Mcal/kg DM) compared to SP70 and SP80. The SP80 roots had the least NFC (P<0.01) and highest amount of fibre. Calcium, phosphorus and vitamin A were negligible post heating. Rate of degradation (c h-1) and ED was highest with SP80 (0.22 and 91.3%; kp=0.03) and lowest with SProots (0.135 and 81.7%). Drying OFSP crop residues and heating roots affected nutrient profiles however, forage degradability improved.
Key words: Sweet potato, vines and leaves, vitamin A, rumen degradation, non-structural carbohydrates.
INTRODUCTION
MATERIALS AND METHODS
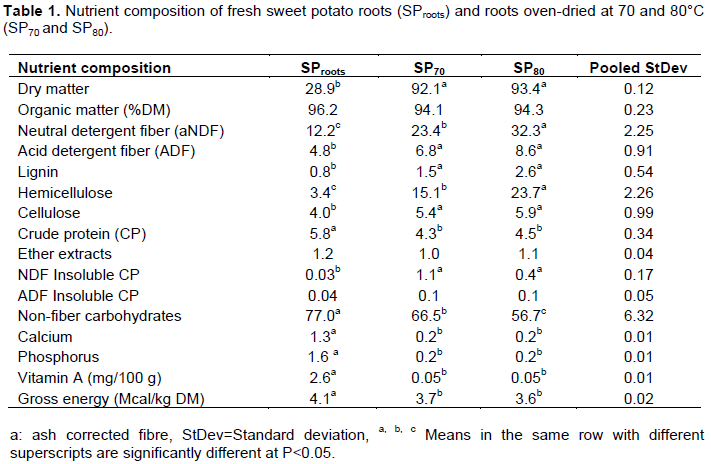
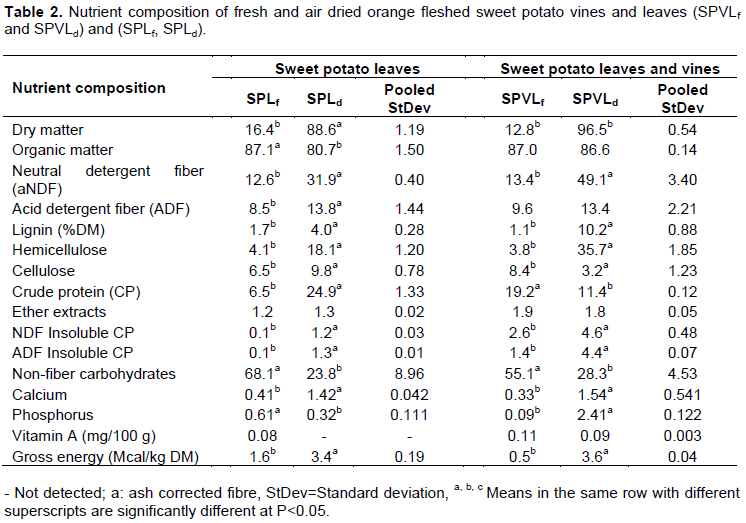
RESULTS AND DISCUSSION
CONCLUSION
CONFLICT OF INTERESTS
REFERENCES
|
Aldrich CG, Rhodes MT, Miner JL, Kerley MS, Paterson JA (1993). The effects of endophyte-infected tall fescue consumption and use of a dopamine antagonist on intake, digestibility, body temperature, and blood constituents in sheep. J. Anim. Sci. 71:158. |
|
|
Allen JC, Corbitt AD, Maloney KP, Butt MS, Truong VD (2012). Glycemic index of sweet potato as affected by cooking methods. Open Nutr. J. 6:1-11. |
|
|
Ahmed M, Akter MS, Eun JB (2010). "Peeling, drying temperatures, and sulphite-treatment affect physicochemical properties and nutritional quality of sweet potato flour," Food Chem. 121(1):112-118. |
|
|
AOAC International (2002). Official methods of analysis. 17th edition. Arlington, Virginia, USA: Association of Official Analytical Chemists Inc. |
|
|
AOAC (1984). Official Methods of Analysis, 14th edition. Association of Official Analytical Chemists, Inc. |
|
|
Bradbury JH, Holloway WD (1988). Chemistry of tropical root crops. Canberra, Australian Centre of International Agriculture Research. |
|
|
Bauman HE, Foster EM (1956). Characteristics of organisms isolated from the rumen of cows fed high and low roughage rations. J. Bacteriol. 71:333-338. |
|
|
Bechoff A (2010). Investigating carotenoid loss after drying and storage of orange-fleshed sweet potato. PhD thesis, University of Greenwich. |
|
|
Becker PM, Yu P (2013). What makes protein indigestible from tissue-related, cellular, and molecular aspects? Mol. Nutr. Food Res. pp. 1-13 Educational Paper Bovell-Benjamin AC (2007). Sweet potato: a review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 52:1-59. |
|
|
Bonte DRL, Picha DH (2000). Carbohydrate-related changes in sweet potato storage roots during development. J. Am. Soc. Hortic. Sci. 125:200-204. |
|
|
Bradhury JH, Hammer BC, Sugani I (1992). Heat stability of trypsin inhibitors in tropical root crops and rice and its significance for nutrition. J. Sci. Food Agric. 58:95-100. |
|
|
Colonna P, Leloup V, Beleon A (1992). Limiting fcators of strach hydrolysis. Eur. J. Clin. Nutr. 46:17-32. |
|
|
Copeland OC (1947). Dehydrated Sweet Potato Meal vs. Ground Shelled Corn for Lactating Dairy Cows. Texas Agric. Exp. Stat. Ann. Rept. 54:28:1941. |
|
|
Dominguez PL (1992). Feeding of sweet potato to monogastrics. In: Machin, D.; Nyvold, S. (Eds.) Roots, roots, plantains and bananas in animal feeding. FAO Animal production and health paper 95, FAO, Roma. |
|
|
Ellong EN, Billard C, Adenet S (2014). Comparison of physiochemical, organoleptic and nutritional abilities of eight sweet potato (Ipomoea batatas) varieties. Food Nutr. Sci. 5:196-211. |
|
|
Engels FM (1989). Some properties of cell wall layers determining ruminant digestion. In: Chesson, A.; Ørskov, E.R. (Eds.), Physio-Chemical Characterization of Plant residues for Industrial and Feed Use. Elsevier Applied Science, London, UK pp. 80-87. |
|
|
Frye JB, Hawkins GE, Henderson HB (1948). The value of winter pasture and sweet potato meal for lactaling dairy cows. J. Dairy. Sci. 31:897-903 |
|
|
Giron HC (1973). Comparison between dry ashing and wet absorption analysis. Absorp. Newsl. 12:28-29. |
|
|
Jung JK, Lee SU, Kozukue N, Levin CE, Friedman M (2011). Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomea batata L.) plants and in home processed roots. J. Food Compos. Anal. 24:29-37. |
|
|
Kariuki JN, Gachuiri CK, Gitau GK, Tamminga S, Bruchem J, Muia JMK, Irungu KRG (1998). Effect of feeding napier grass, lucerne and sweet potato vines as sole diets to dairy heifers on nutrient intake, weight gain and rumen degradation. Livest. Prod. Sci. 55:13-20. |
|
|
Kirana KS, Padmaja G (2003): Inactivation of trypsin inhibitors in sweet potato and taro roots during processing. Plant Foods Hum. Nutr. 58:153-163. |
|
|
Kohyama K, Nishinari K (1992). Cellulose derivatives effects on gelatinization and retrogration of swete potato starch. J. Food Sci. 57:128-131. |
|
|
Laurie SM (2010). Agronomic performance, consumer acceptability and nutrient content of new sweet potato varieties in South Africa. PhD thesis. University of Free State, Bloemfontein. |
|
|
Lewthwaite SL, Suton KH, Triggs CM (2010). Free sugar composition of sweet potato cultivars after storage. New Zealand J. Crop Hortic. Sci. 25:33-41. |
|
|
Lin YH (1989). Relationship between trypsin – inhibitor activity and water – soluble protein and cumulative rainfall in sweet potato. J. Am. Soc. Hortic. Sci. 114:814-818. |
|
|
Maeshima M, Sasaki T, Asahi T (1985). Characterization of major proteins in sweet potato tuberous roots. Phytochemistry. 24:1899-1902 |
|
|
Manz U, Philipp K (1981). A method for the routine determination of Tocopherols in animal feed and human foodstuffs with the aid of high performance liquid chromatography. Int. J. Vit. Nutr. Res. 51:342-348. |
|
|
Mauron J (1990). Influence of processing on protein quality. J. Nutr. Sci. Vitaminol. 36:S57-S69. |
|
|
Meade SJ, Reid EA, Gerrard JA (2015). The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 88:904-922. |
|
|
Mertens DR (1994). Regulation of forage intake. In: Forage Quality, Evaluation and Utilization; American Society of Agronomy, WI, USA pp. 450-493. |
|
|
Mohanraj R, Sivasankar S (2014). Sweet Potato (Ipomoea batatas [L.] Lam) - A valuable medicinal food: A Review. J. Med. Food 17:733-741. |
|
|
Nafeesa A, Falade KO, Akingbala JO (2012). Effect of Cultivar on Quality Attributes of Sweet Potato Fries and Crisps. Food Nutr. Sci. 3:224-232. |
|
|
NRC (2001). Nutrient Requirements of Dairy cattle: Seventh revised edition. Subcommittee on Dairy Cattle Nutrition, Committee on Animal Nutrition and Board on Agriculture and Natural Resources. National Academy Press, Washington, D.C |
|
|
Ørskov ER, McDonald I (1979). The estimation of protein degradability in the rumen from incubation measurements weighed according to rate of passage. J. Agric. Sci. 92:499-503. |
|
|
Ørskov ER, Hovell FD, Mould F (1980). The use of the nylon bag technique for the evaluation of feedstuffs. Trop. Anim. Prod. 5(3):195-213. |
|
|
Peters D (2008). Assessment of the Potential of Sweet Potato as Livestock Feed in East Africa: Rwanda, Uganda, and Kenya. A report presented to The International Potato Centre (CIP) in Nairobi. |
|
|
Phesatcha K, Wanapat M (2013). Performance of lactating dairy cows fed a diet based on treated rice straw and supplemented with pelleted sweet potato vines. Trop. Anim. Health Prod. 45:533-538. |
|
|
Rekha MR, Padmaja G (2002). Alpha amylase inhibitor changes during processing of sweet potato and taro roots. Plant Foods Human Nutr. 57:285-294. |
|
|
Robertson JB, Van Soest PJ (1981). The detergent system of analysis and its application to human foods. In: James, W.P.T.; Theander, O. (Eds.), The analysis of dietary fibre in food. Dekker Basic and Clinical Nutr. 3:158-276. |
|
|
Russell JB, O'Connor JD, Fox DG, Van Soest PJ, Sniffen CJ (1992). A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 70:3551-3561. |
|
|
Statistical Analyses Systems (2010). Version 9.3, SAS institute Inc, Cary, N.C. |
|
|
Senanayake SA, Ranaweera KKDS, Gunaratne A, Bamunuarachchi A (2013). Comparative analysis of nutritional quality of five different cultivars of sweet potatoes (Ipomea batatas (L) Lam) in Sri Lanka. Food Sci. Nutr. 1:284-291. |
|
|
Smit CJ (2015). Effects of Sweet Potato Forage Meals on Protein and Energy Supply, Beta-Carotene and Blood Glucose Content of Dairy Cattle Milk. MSc (Agric) Thesis, University of South Africa, South Africa |
|
|
Sun H, Mu T, Xi L, Zhang M, Chen J (2014a). Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 156:380-389. |
|
|
Sun H, Mu T, Xi L, Song Z (2014b). Effects of domestic cooking methods on polyphenols and antioxidant activity of sweet potato leaves. J. Agric. Food Chem. 62:8982-8989. |
|
|
USDA NAL (2015). https://fnic.nal.usda.gov/food-composition. |
|
|
Van Hal M (2000). Quality of sweet potato flour during processing and storage. Food Rev. Int. 16:1-37. |
|
|
Van Soest PJ (1994). Nutritional Ecology of the Ruminant. Cornell University, USA. 476 pp. |
|
|
Van Soest PJ, Robertson JB, Lewis BA (1991). Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583-3597. |
|
|
Walter WM, Purcell AE (1986). Protein of sweet potato. ACS Symposium Series 312,234-248. In: Ory R. L (Editor). Plant Proteins: Appl. Biol. Effects Chem. 19:234-248. |
|
|
Walter WM, Purcell AE, Hoover AE (1976). Changes in amyloid carbohydrates during preparation of sweet potato flakes. J. Food Sci. 41:1374-1377. |
|
|
Xi L, Sun H, Mu T, Zhang M, Chen J (2015). The antioxidant activity in vitro and processing stability of sweet potato leaf polyphenols. J. Chinese Institute Food Sci. Technol. 15, 147-156. |
|
|
Xu J, Su X, Lim S, Griffin J, Carey E, Katz B, Tomich J, Scott Smith J, Wang W (2015). Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 186:90-96. |
|
|
Yadang G, Mbome IL, Ndjouenkeu R (2013). Changes in amylase activity, hot-paste viscocity and carbohydrates during natural fermentation of sweet potato (Ipomoea batatas). Afr. J. Food Sci. Technol. 4:188-194. |
|
|
Zhang Z, Corke H (2001). Trypsin inhibitor activity in vegetative tissue of sweet potato plants and its response to heat treatment. J. Sci. Food Agric. 81:1358-1363. |
|
|
Zhi-feng F, Tu Z, Zhang L, Wang H, Wen Q, Huang T (2016). Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 15:11-18. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0